 There are many different methods in use today to acquire data on a mass spectrometer, but few have generated as much buzz in recent years as SWATH technology. First reported 5 years ago by Ruedi Aebersold and his group1, SWATH® Acquisition on a TripleTOF® instrument has rapidly become one of the premier acquisition strategies for identification and quantitation of complex samples. But what exactly is SWATH and why is it so powerful? In order to answer these questions, let’s first take a step back and look at the larger picture.
There are many different methods in use today to acquire data on a mass spectrometer, but few have generated as much buzz in recent years as SWATH technology. First reported 5 years ago by Ruedi Aebersold and his group1, SWATH® Acquisition on a TripleTOF® instrument has rapidly become one of the premier acquisition strategies for identification and quantitation of complex samples. But what exactly is SWATH and why is it so powerful? In order to answer these questions, let’s first take a step back and look at the larger picture.
Mass spectrometry can be used to analyze all sorts of compounds and answers all kinds of questions. Depending upon the end goal—whether it is for profiling for known compounds, identification of unknown compounds, quantitation, or characterization—the specific way that a mass spectrometer is set up to scan and target ions for analysis will vary.
For complex samples where the goal is to identify as many compounds as possible, a method called Data Dependent Acquisition (DDA) has been the most widely used strategy for over 20 years, particularly when coupled with liquid chromatography (LC). In the DDA approach, as compounds elute from an LC column and enter the mass spectrometer, the instrument quickly scans them all and then chooses a subset of those compounds, or precursors, for further analysis. The instrument then focuses on each one of those precursors and fragments it generating MS/MS data. From the MS/MS data, the identities of the compounds can be determined. The selection of which ions get fragmented is “dependent” upon some criteria previously set-up in the method and is usually sorted based on their abundance. So for example, the method might specify that only the 20 most abundant ions get fragmented for each cycle. This cycle can last a few seconds while all 20 precursors are fragmented, and then the cycle will start over with a new set of precursors. Over the course of the entire LC run, MS/MS data is generated from the 20 most abundant precursors that happen to be eluting in each cycle. Because the subset of precursors selected is dependent upon the precise make-up of the mixture of compounds entering the instrument at that very moment in time, the DDA method is somewhat random. As a result, run-to-run reproducibility can suffer, the same compounds will not be selected for MS/MS in each run. In addition, especially in more complex samples, there are a lot more than 20 compounds eluting at any point in time. This intensity based, one-by-one sampling results in a lot of important compounds never get selected and therefore go unidentified.
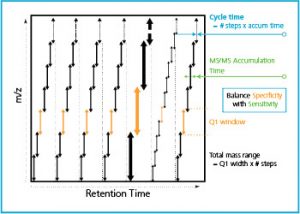 In contrast to traditional DDA, SWATH is a Data Independent Acquisition strategy (DIA). With SWATH, every detectable analyte is fragmented, not just a subset. For each cycle, the instrument focuses on a narrow mass window of precursors and acquires MS/MS data from all precursors detected within that window. This mass window is then stepped across the entire mass range, systematically collecting MS/MS data from every mass and from all detected precursors.
In contrast to traditional DDA, SWATH is a Data Independent Acquisition strategy (DIA). With SWATH, every detectable analyte is fragmented, not just a subset. For each cycle, the instrument focuses on a narrow mass window of precursors and acquires MS/MS data from all precursors detected within that window. This mass window is then stepped across the entire mass range, systematically collecting MS/MS data from every mass and from all detected precursors.
SWATH Acquisition results in a truly comprehensive data set. Contrary to DDA where only a subset of precursors is sent for fragmentation and can be identified, in SWATH every ion is fragmented and can be identified. The fragmentation is “independent” of any characteristic that the ion possesses (such as abundance). Additionally, because the entire precursor mass range is fragmented, no gaps in the data are observed, and run-to-run reproducibility is extremely high.
 Because the SWATH method selects a mass window for fragmentation (rather than a single mass as in DDA), the MS/MS data that is generated contains fragment ions from all precursors found in that window. Multiple compounds are fragmented all at once. This makes the MS/MS data more complex than in traditional DDA and places a tremendous burden on the specifications of the instrument used for acquisition. While good results can be achieved for DDA even using lower performing instruments, the same is not true for DIA. For successful DIA, high resolution in MS/MS mode becomes extremely important. With such a complex MS/MS spectrum, it is imperative to keep the fragment peaks as highly resolved as possible so there is no chance of overlap which would interfere with accurate identification. Additionally, because multiple precursors can be fragmented simultaneously, dynamic range is extremely important. Fragment ions from low abundant analytes can be obscured in the presence of fragments from highly concentrated analytes, and so the instrument must be capable of high dynamic range to account for this. Fast MS/MS acquisition is also extremely important. The instrument must be able to take many steps to cover entire precursor mass range before new peaks elute into the mass spectrometer, or they will be gone before the next cycle. And of course, sensitivity is paramount in order to detect even the lowest level analytes. These attributes are indivisible. Having great resolution without speed, or sacrificing sensitivity for better dynamic range is simply not good enough for DIA.
Because the SWATH method selects a mass window for fragmentation (rather than a single mass as in DDA), the MS/MS data that is generated contains fragment ions from all precursors found in that window. Multiple compounds are fragmented all at once. This makes the MS/MS data more complex than in traditional DDA and places a tremendous burden on the specifications of the instrument used for acquisition. While good results can be achieved for DDA even using lower performing instruments, the same is not true for DIA. For successful DIA, high resolution in MS/MS mode becomes extremely important. With such a complex MS/MS spectrum, it is imperative to keep the fragment peaks as highly resolved as possible so there is no chance of overlap which would interfere with accurate identification. Additionally, because multiple precursors can be fragmented simultaneously, dynamic range is extremely important. Fragment ions from low abundant analytes can be obscured in the presence of fragments from highly concentrated analytes, and so the instrument must be capable of high dynamic range to account for this. Fast MS/MS acquisition is also extremely important. The instrument must be able to take many steps to cover entire precursor mass range before new peaks elute into the mass spectrometer, or they will be gone before the next cycle. And of course, sensitivity is paramount in order to detect even the lowest level analytes. These attributes are indivisible. Having great resolution without speed, or sacrificing sensitivity for better dynamic range is simply not good enough for DIA.
SWATH is not the first DIA approach to ever be developed. Indeed, there have been other DIA techniques used on other instruments in the past. So what makes SWATH so special? Well, many things actually. First and foremost, SWATH Acquisition can only be performed on a high-performance mass spectrometer. As we mentioned previously, the burdens placed upon instrument specifications for a DIA approach are extremely high, with no room for compromise. The TripleTOF 6600 system has up to 5 orders of magnitude in MS/MS mode to enable the detection of the lowest intensity fragments even when surrounded by ions 100,000x more abundant. Additionally, The TripleTOF is the fastest scanning, highest resolution MS/MS data generating instrument for DIA available, capable of generating 100 MS/MS spectra with 30k resolution per second. Other instruments may be capable of high resolution, high scan speed, high sensitivity, and/or high dynamic range, but not all at the same time which is imperative for a successful DIA approach.
SWATH also has other innovations too. For example, the range of the precursor window is adjusted depending upon how crowded it may be. With the TripleTOF 6600, up to 200 SWATH Variable Width Windows can be sequentially stepped across the mass range per cycle. This enables narrower windows to be used where analyte density is greatest – as low as 5-6Da – and wider windows where analyte density is sparse. This allows for better data quality without sacrificing speed.
With SWATH Acquisition, there is virtually no method development. The instrument is programmed to step across the entire mass range and fragment everything it detects. Thus, every acquisition method, regardless of the sample, is identical, because the instrument acquires data on everything. Every time.
What about data analysis? Most DDA and other DIA techniques use some form of database search strategy. However, analyzing DIA datasets with traditional database search strategies is challenging due to the fact that each MS/MS spectrum may derive from the fragmentation of multiple precursors all found within the mass window. This makes identification difficult. To get around this, SWATH employs a library matching approach to extract the data in a targeted way. To prepare the library, previous DDA experiments are performed in order to generate MS/MS spectra that can be identified with high confidence using traditional database search methods. Then these actual spectra are saved into a library. This can be repeated over and over to generate the most comprehensive library possible. Fractionation is often performed to help generate a deep and comprehensive library. Once the library is generated, it can be used over and over. During SWATH data processing, an algorithm systematically extracts all the fragment ions for a peptide from a SWATH window of the correct precursor mass. These groups of XICs (peak groups) are then scored using a range of criteria to determine which most likely represented the targeted peptide. Thus, specific peptides can be identified/confirmed and quantified using the targeted data extraction strategy.
But remember, SWATH acquisition is not just for identification. With SWATH, compounds are not only identified, but they are also quantified. The quantitation is comparable to MRM techniques which are considered the gold standard for quantitation by mass spectrometry. The ability to comprehensively identify and accurately quantify compounds in very complex samples over a large dynamic range and in an extremely reproducible fashion opens up a world of possibilities and fills a much-needed void in comparative research studies.
For more information watch the 5-minute video MS/MS ALL with SWATH Acquisition, or visit our SWATH page. For proteomics researchers, join the SWATH discussion groups on the SCIEX Community. Stay tuned for our next blog in this series where we discuss how SWATH can be used for different types of samples and applications.
References
1. Targeted Data Extraction of the MS/MS Spectra Generated by Data-independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Ludovic C. Gillet,Pedro Navarro,Stephen Tate,Hannes Röst,Nathalie Selevsek,Lukas Reiter, Ron Bonner,and Ruedi Aebersold, Mol Cell Proteomics. 2012 Jun; 11(6). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3433915/






 Contact Support
Contact Support
0 Comments