 Fast, accurate, and robust solution for routine commercial cannabis testing
Fast, accurate, and robust solution for routine commercial cannabis testing
As the world debates cannabis legalization for therapeutic applications and recreational use, the trends are shifting. Medicinal use of cannabis is legal in an increasing number of countries worldwide, including 33 states and the District of Columbia in the United States. Uruguay was the first country in the world to legalize the sale, cultivation, and distribution of cannabis in 2013. In the United States, Washington and Colorado were the first to fully legalize cannabis in 2012. By the end of 2019, 10 states have legalized recreational use for adults over the age of 21, with 64% of Americans favoring the move.
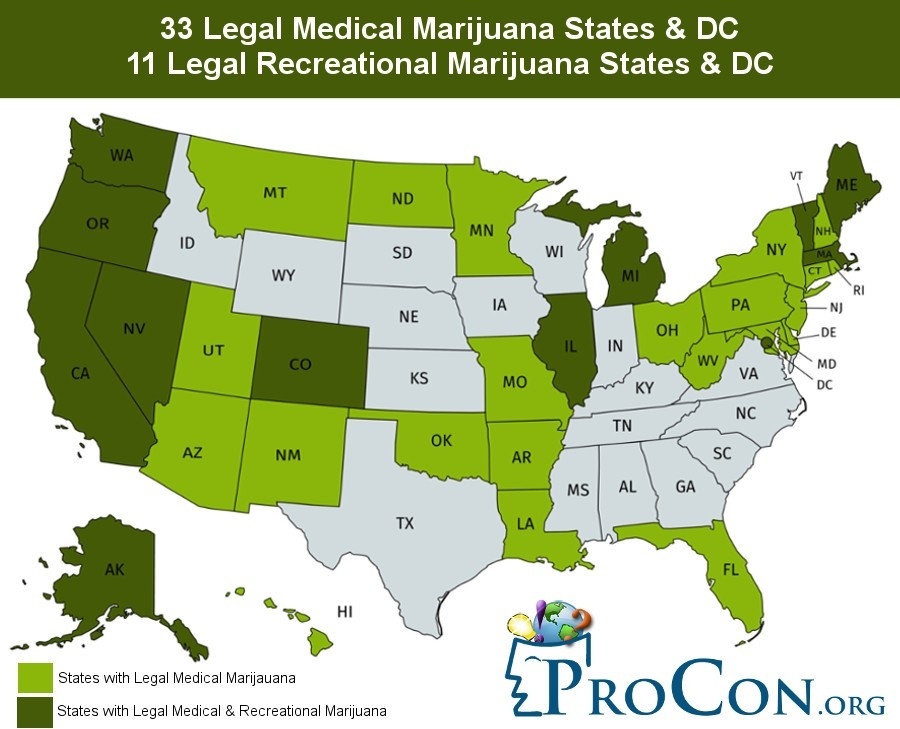
Source: ProCon.org
In the past, cannabis agriculture has been completely unregulated, with distribution driven by unlawful dispensaries. However, with legalization, this industry is rapidly blooming. While the trend is celebrated by campaigners worldwide, concerns around public safety are increasing. For one thing, not all cannabis that hits the shelves is safe. There’s a possibility that these products are laced with contaminants, or that they are so potent that they are not safe for consumption.
Cannabis testing for safety
Accurate testing is a critical piece to help ensure consumer safety. Your lab needs robust methods for routine commercial analysis to test your marijuana and hemp samples for:
- Pesticides, including insecticides, fungicides and rodenticides
- Potency of cannabinoids including tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG) and cannabinol (CBN)
- Mycotoxins (such as aflatoxin B1 and B2 or ochratoxin A) and mold (such as fusarium or aspergillus)
- Terpene profiling
With public safety a primary concern for regulators, it comes as no surprise that regulators are clamping down on dispensaries. With legalization in the United States defined by each state, there is varying legislation indicating what needs to be monitored and at what levels. The legislation also specifies action limits or maximum residue limits (MRLs) that must not be exceeded. Screening has become a prerequisite, and cannabis testing labs are feeling the strain.
- The first challenge is supply and demand—there aren’t enough labs to keep up with the volume of samples. Labs need to increase throughput with a fast and reliable analytical technique. The ideal solution would involve a single instrument capable of analyzing all chemical residues at the lowest possible limit to analyze samples for potency, pesticides, mycotoxins and terpenes.
- Secondly, cannabis testing is intrinsically complex—the Cannabis sativa plant contains hundreds of chemical entities across a vast range of concentrations. The major target compounds are tetrahydrocannabinol (THC) and cannabidiol (CBD). Your lab probably has to quantify many residues with dirty matrices and a lot of sample variation, since cannabis can be ingested by mouth, topically or by inhalation. Labs need rugged instrumentation to manage a complex workflow and solid application support from the instrument vendor.
- Labs face inconsistent maximum residue limits—so they require a pre-defined compound list for targeted analysis that aligns with legislation action limits or acceptable maximum residual levels. Labs also need access to the techniques that will enable them to perform the required targeted analysis.

Where mass spectrometry plays a role in cannabis testing technology
Early liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods were developed primarily for residue analysis of pesticides, herbicides, plant growth regulators and mycotoxins. These methods cover a wider range of compounds than gas chromatography-mass spectrometry (GC/MS) methods and offer more sensitivity, robustness and productivity. In later years, we’ve seen other LC-MS/MS methodologies and applications developed that easily and accurately quantify cannabinoids. This includes high-resolution accurate mass (HRMS) techniques that prove to be useful in chemovar identification, and also analysis using QTRAP® systems, which offer the increased specificity of MRM3 scans.
We understand that cannabis poses its own set of unique testing challenges. From setting up a lab and developing methods for the analysis of cannabis plants and products, to keeping up with emerging regulations, it’s an ever-changing landscape. Whether you’re in a reference lab, an in-house testing lab or supporting law enforcement, you need the right technology, method and partner to drive exceptional return on investment.
Click the banner above to access the compendium on global marijuana and hemp trends and landmark analytical research on the following topics:
- Potency of cannabinoids including CBD and CBN
- Pesticides like etridiazole and diazinon
- Mycotoxins such as aflatoxin
- Toxins like vitamin E and vitamin E acetate in vaping oils
- Forensics of DUID blood samples
 About me, Paul Winkler
About me, Paul Winkler
I play an active role in supporting cannabis (marijuana and hemp) applications at SCIEX. I have more than 30-years of experience owning and operating contract labs in the environmental and bioanalytical markets. I’ve been part of the SCIEX family since 2011; started as a field application chemist and today I’m a market development manager for the North America team; helping customers like you with your analytical challenges and educating the market on the many possibilities of LC-MS/MS systems is my passion.






 Contact Support
Contact Support
0 Comments