 In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual. In fact, recent applications of serial PK microsampling have demonstrated that sufficient data could be obtained for a comparison of Day 1 and Day 5 profiles in the same mouse when sampling only 8 µL of blood.1 This equates to a 95% reduction in the total number of mice needed for a five-day study when compared to discrete sampling approaches using > 250 µL blood. A further benefit of microsampling is the economic impact since fewer animals to dose and sample translates to less compound consumption and personnel required. Finally, microsampling techniques allow refinement of the sampling process as removal of smaller blood volumes allows less invasive tail vein sampling as opposed to cardiac puncture.
In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual. In fact, recent applications of serial PK microsampling have demonstrated that sufficient data could be obtained for a comparison of Day 1 and Day 5 profiles in the same mouse when sampling only 8 µL of blood.1 This equates to a 95% reduction in the total number of mice needed for a five-day study when compared to discrete sampling approaches using > 250 µL blood. A further benefit of microsampling is the economic impact since fewer animals to dose and sample translates to less compound consumption and personnel required. Finally, microsampling techniques allow refinement of the sampling process as removal of smaller blood volumes allows less invasive tail vein sampling as opposed to cardiac puncture.
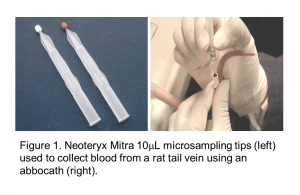
 Despite the numerous benefits associated with sampling low blood volume (≤ 20 µL), bioanalytical challenges exist depending upon the microsampling technique used. In the case of dried-blood-spot (DBS) sampling, the hematocrit (HCT) of the blood affects its viscosity, giving rise to different-sized blood spots that may result in low quality drug concentration data when using a fixed-diameter sub-punch.2 While capillary microsampling (CMS) techniques circumvent the HCT-effect exhibited by DBS and allow analysis of both blood and plasma from a single sample, collection and processing is much more tedious.3-4 Further, drugs that exhibit non-specific binding to glass or require matrix stabilization can be problematic. A recent and promising alternative to both DBS and CMS is Volumetric Absorptive Microsampling (VAMS), wherein a fixed, accurate volume of blood is absorbed by capillary action onto 10 µL or 20 µL hydrophilic polymeric porous tips anchored to a handle by a plastic pin (Figure 1). Manufactured by Neoteryx under the commercial name Mitra™, the technology promises the benefits of DBS sampling while overcoming potential HCT effects, simplifying sample collection and processing, and reducing extraction difficulties associated with CMS.5, 6
Despite the numerous benefits associated with sampling low blood volume (≤ 20 µL), bioanalytical challenges exist depending upon the microsampling technique used. In the case of dried-blood-spot (DBS) sampling, the hematocrit (HCT) of the blood affects its viscosity, giving rise to different-sized blood spots that may result in low quality drug concentration data when using a fixed-diameter sub-punch.2 While capillary microsampling (CMS) techniques circumvent the HCT-effect exhibited by DBS and allow analysis of both blood and plasma from a single sample, collection and processing is much more tedious.3-4 Further, drugs that exhibit non-specific binding to glass or require matrix stabilization can be problematic. A recent and promising alternative to both DBS and CMS is Volumetric Absorptive Microsampling (VAMS), wherein a fixed, accurate volume of blood is absorbed by capillary action onto 10 µL or 20 µL hydrophilic polymeric porous tips anchored to a handle by a plastic pin (Figure 1). Manufactured by Neoteryx under the commercial name Mitra™, the technology promises the benefits of DBS sampling while overcoming potential HCT effects, simplifying sample collection and processing, and reducing extraction difficulties associated with CMS.5, 6
 Regardless of the microsampling technique applied, adequate sensitivity in lieu of reduced sample volume represents one of the major hurdles facing the development of a supporting bioanalytical assay. This sensitivity challenge is further exacerbated in the case of biotherapeutics given their propensity for multiple-charging by electrospray ionization and the formation of numerous fragment ions under MS/MS conditions. In this blog, I examine the sensitivity and assay performance of the SCIEX 6500+ for supporting the evaluation of VAMS technology and its application to the generation of a PK-profile from a single rodent dosed subcutaneously with the GLP-1 agonist Exenatide, a 4.2 kDa biotherapeutic used in the treatment of type II diabetes.
Regardless of the microsampling technique applied, adequate sensitivity in lieu of reduced sample volume represents one of the major hurdles facing the development of a supporting bioanalytical assay. This sensitivity challenge is further exacerbated in the case of biotherapeutics given their propensity for multiple-charging by electrospray ionization and the formation of numerous fragment ions under MS/MS conditions. In this blog, I examine the sensitivity and assay performance of the SCIEX 6500+ for supporting the evaluation of VAMS technology and its application to the generation of a PK-profile from a single rodent dosed subcutaneously with the GLP-1 agonist Exenatide, a 4.2 kDa biotherapeutic used in the treatment of type II diabetes.
Sampling, Recovery and HCT Effect Mitra sampling was most effective when the hydrophilic tip just contacted the blood surface, allowing rapid absorption by capillary action (2-4 sec). An additional two seconds was added to ensure complete saturation of the tip. Following sampling, tips were air dried for ca. 30 minutes, then a further 24 hr in the presence of desiccant. Tips were removed by angular displacement against the inside of a 96-well plate and extracted as noted in Figure 2. Exenatide extracted from blood with HCT levels between 20% and 66% referenced against plasma (0% HCT) indicated minimal recovery bias between average blood yields and plasma. Further, recovery differences within the normal rodent HCT range of 34% – 48% were largely insignificant (Figure 3). The actual volume of rodent blood absorbed by the 10 µL Mitra tip was determined gravimetrically as a function of % HCT to assess the latters impact on sampling volume. Precision and accuracy data presented in Table 1, based on 12 replicate measurements for each HCT level, revealed an absence of sampling volume bias.
Mitra sampling was most effective when the hydrophilic tip just contacted the blood surface, allowing rapid absorption by capillary action (2-4 sec). An additional two seconds was added to ensure complete saturation of the tip. Following sampling, tips were air dried for ca. 30 minutes, then a further 24 hr in the presence of desiccant. Tips were removed by angular displacement against the inside of a 96-well plate and extracted as noted in Figure 2. Exenatide extracted from blood with HCT levels between 20% and 66% referenced against plasma (0% HCT) indicated minimal recovery bias between average blood yields and plasma. Further, recovery differences within the normal rodent HCT range of 34% – 48% were largely insignificant (Figure 3). The actual volume of rodent blood absorbed by the 10 µL Mitra tip was determined gravimetrically as a function of % HCT to assess the latters impact on sampling volume. Precision and accuracy data presented in Table 1, based on 12 replicate measurements for each HCT level, revealed an absence of sampling volume bias.
Sensitivity Early attempts at using an API 5500 for the development of the VAMS exenatide assay led to a number of challenges due to sensitivity limitations. Extracts required concentrating, which led to non-specific binding and a strong matrix effect impacting ionization efficiency. In contrast, by leveraging the sensitivity gains of the 6500+, it was unnecessary to concentrate extracts, and in fact, a 36-fold dilution factor could be realized. This dilution factor expedited the sample preparation process since the solution extract could simply be diluted to initial mobile phase conditions and injected. Further, since matrix effect was successfully diluted out, rapid run times of 2 minutes could be achieved, as it was unnecessary to chromatographically resolve ion suppressors. As outlined in Figure 4, a 20-fold response gain, or 7-fold gain in S/N, was achieved on the 6500+ for the same sample of exenatide extracted at the LOQ and injected on the API 5500.
Early attempts at using an API 5500 for the development of the VAMS exenatide assay led to a number of challenges due to sensitivity limitations. Extracts required concentrating, which led to non-specific binding and a strong matrix effect impacting ionization efficiency. In contrast, by leveraging the sensitivity gains of the 6500+, it was unnecessary to concentrate extracts, and in fact, a 36-fold dilution factor could be realized. This dilution factor expedited the sample preparation process since the solution extract could simply be diluted to initial mobile phase conditions and injected. Further, since matrix effect was successfully diluted out, rapid run times of 2 minutes could be achieved, as it was unnecessary to chromatographically resolve ion suppressors. As outlined in Figure 4, a 20-fold response gain, or 7-fold gain in S/N, was achieved on the 6500+ for the same sample of exenatide extracted at the LOQ and injected on the API 5500.

Assay Performance and PK Profile Generation
Calibrants extracted across a 500-fold concentration range exhibited a linear response with a correlation coefficient of 0.9966, indicating a consistent response factor and therefore extraction recovery (Figure 5). Between-run precision and accuracy based on three separate extraction occasions met all acceptance criteria (Table 2), as did specificity, matrix effect, matrix factor and dilution integrity assessments. Further, low and high QCs prepared in blood at HCT levels between 20% and 66% demonstrated back-calculated concentrations within ±15% when determined against calibrants prepared in blood at an HCT level of 44%, indicating the lack of HCT effect impacting sampling or extract recovery. The application of the VAMS technique for the determination of exenatide PK profiles from three different rodents demonstrated excellent agreement between individuals. Samples were collected in triplicate for each time point, resulting in only 240 µL of blood being drawn from each subject. Cmax was achieved after 30 minutes with an average concentration of 173.5 ng/mL (13% CV); after 6 hr, 98% of exenatide was eliminated from the bloodstream.


Conclusions
Volumetric absorptive microsampling offered a simplified workflow for sample collection and transfer without the need for centrifugation, matrix transfer, frozen sample storage or sample sub-punching. Further, no HCT effect was noted. However, as with all microsampling techniques, volume limitations challenged achievable detection limits, and therefore these workflows are best supported by the most sensitive MS/MS systems available.
References
1. Korfmacher et al., Bioanalysis 7(4) 449-461 (2015)
2. Youhnovski et al., Bioanalysis 2(8) 1501-1513 (2010)
3. Nilsson et al., Bioanalysis 5(6) 731-738 (2013)
4. Dillen et al., Bioanalysis 6(3) 293-306 (2014)
5. Spooner et al., Bioanalysis 7(6) 653-659 (2015)
6. Ye et al., Bioanalysis 9(4) 349-357 (2017)
* The author gratefully acknowledges the collaborative efforts of his R&D colleagues at Algorithme Pharma, an Altasciences Company.






 Contact Support
Contact Support
0 Comments