SWATH® Acquisition improves metabolite coverage over traditional data dependent techniques for untargeted metabolomics
A data independent acquisition technique employed on the TripleTOF® 6600 System
Zuzana Demianova1; Cyrus Papan1; Joerg Dojahn1; Baljit K. Ubhi2
1SCIEX, Germany; 2SCIEX, USA
Abstract
SWATH® Acquisition, a data independent acquisition (DIA) workflow is well adopted in quantitative discovery proteomics, but still not commonly used in discovery metabolomics. Here, it is described how SWATH Acquisition enables the identification of a higher number of metabolites for untargeted metabolomics workflows compared to traditional data dependent acquisition (DDA) approaches, thus enabling a broader profile of the metabolome. The results show that SWATH Acquisition using variable windows improves metabolite coverage using the Accurate Mass Metabolite Spectral Library (AMMSL) compared with the traditional DDA approach.

Introduction
SWATH® Acquisition, a data independent acquisition (DIA) workflow is well adopted in quantitative discovery proteomics1, but still not commonly used in discovery metabolomics. Traditional data dependent acquisition (DDA) techniques are heavily employed in the field of metabolomics and workflows on the mass spectrometers have been adapted so that as much data as possible can be captured. This has led to a two-injection workflow in the community; one injection to collect the MS and mine the precursor data and a second for the MS/MS to confirm the metabolite identification. Researchers were limited by the speed of their mass spectrometers because they could not scan fast enough to capture both the MS and MS/MS data in a single injection. Also, the stochastic nature of data dependent workflows often means MS/MS of low abundant metabolites are often missed.
The TripleTOF 6600 System allows both the MS and MS/MS data to be collected in a single injection allowing the collection of a digitized map of every detectable metabolite in the sample - meaning no need to go back and re-run a sample but just re-mine the data as the hypothesis changes. Here, it is demonstrated how SWATH Acquisition enables the identification of a higher number of metabolites for untargeted metabolomics workflows2 compared to traditional data dependent acquisition (DDA) approaches thus enabling a broader profile of the metabolome.2 Results show that SWATH Acquisition using variable Q1 window acquisition3 improves metabolite coverage using the Accurate Mass Metabolite Spectral Library (AMMSL) compared with the traditional DDA approach.
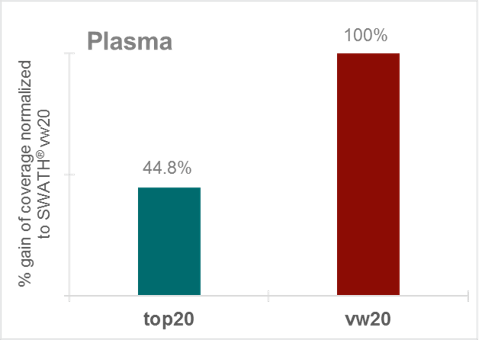
Figure 1. Gain in metabolite coverage in plasma extracts. Over 55% gain in metabolite coverage with SWATH Acquisition with 20 variable width windows over top20 DDA acquisition.
Benefits of the SWATH® Acquisition workflow for metabolomics applications
- Full scan MS and MS/MS of every single metabolite in a sample in a single injection
- Comprehensive identification and quantitation of the metabolites in the sample
- No method development required
- Permanent digital record of the metabolome of your sample
- Narrower Q1 isolation windows provide improved data quality through increased specificity3
- SWATH Variable Window Calculator4 can be used to optimize the Q1 isolation window pattern for the matrix of interest, to achieve the right balance of metabolite coverage and specificity.
Methods
Sample preparation: Human urine and commercially available human plasma were processed according to standard extraction protocols. Urine was diluted with water at a ratio of 1:4 (v/v) and centrifuged for prior analysis, whilst plasma was extracted 1:4 (v/v) with ice-cold methanol allowing for protein precipitation.
Chromatography: Separation was performed on an Agilent Technologies 1290 Infinity II using an Acquity BEH C18 column with dimensions; 100mm x 2.1 mm ID, 1.7 µm (Waters, Milford, USA) using a flow rate 200 µL/min. A gradient was employed from 1-10 minutes from 2-98% of 0.1% formic acid in acetonitrile, total length of LC separation was 14 minutes and column oven temperature was set to 40oC. Injection volume was 5 µL for both type of samples.
Mass spectrometry: The SWATH Acquisition and DDA experiments were acquired on a TripleTOF 6600 System in positive ion mode. The source settings were Curtain Gas 35 psi, GS1 40 psi, GS2 40 psi, ISVF 5500 V, Source temp. 600°C, Declustering Potential 80 V. MS/MS was acquired with a collision energy was 30 V with 15 V spread.
For the DDA acquisition, the top 5, 10, 15, 20 and 25 precursor ions per cycle were selected for MS/MS, described hereon in as top5, top10, top15, top 20 and top25 (Table 1). For SWATH Acquisition, the number of Q1 windows was varied using 15, 20 and 30 windows, both fixed window (fw) and variable window (vw) widths. All these settings were used to test which parameters resulted in the highest number of identifications and coverage of metabolites in plasma and urine extracts.
The Top20 DDA acquisition method was used to calculate variable windows using a SWATH Variable Window Assay Calculator version 1.1 with minimum window size 3 Da.4 Example graphs illustrating the correlation of the SWATH Acquisition window sizes versus the precursor ion density is presented in Figure 2.
Data processing: Data was processed using SCIEX OS Software or MasterView™ Software and the Accurate Mass Metabolite Spectral Library (AMMSL) using search settings accordingly: candidate search algorithm, results sorted by Purity (for DDA data) or Fit (for SWATH Acquisition data). A combined score (isotope distribution pattern, fragmentation pattern, mass error) of >70% was used to evaluate the confidence in the metabolite identification.
Figure 2. Variable Q1 window widths explored across sample matrices. To achieve better specificity in complex matrices, smaller Q1 windows are desirable especially in the m/z dense regions where many analyte precursors are found. The m/z density histograms constructed from the TOF MS data for the sample of interest (blue line) can be used to a construct variable sized window pattern (red line) using the SWATH Variable Window Calcluator.4 The goal is to equalize the density of precursors in each of the isolation windows across the m/z range. A) A urine sample with 15 variable windows (vw) and B) with vw30 highlighting more specificity through smaller Q1 windows in the densest regions of the data; C) A plasma sample with vw15 and D) with vw30.
Table 1. Experimental comparison. (Top) DDA acquisition MS method settings. (Bottom) SWATH Acquisition MS settings for fixed windows (fw) and variable windows (vw) size.
Data dependent acquisition
In the first part of the study, the traditional DDA acquisition strategy was evaluated by comparing the number of precursors selected for MS/MS analysis. An identical accumulation time of 25ms was used across these DDA experiments to be able to compare the data. The coverage of these different methods was evaluated by matching the metabolites to the spectral library (AMMSL) which contains over 550 exogenous and endogenous metabolites to a human plasma extract (Figure 3). A library score of 70% and above was used as the cutoff criteria for a high quality confirmed metabolite match.
The data demonstrate a significant improvement of metabolite coverage at the MS/MS level when comparing the top5 to the top25 DDA method (Figure 3). In Figure 3, an increase in metabolite coverage over 100% was observed in plasma extracts by increasing the number of selected precursor ions for DDA acquisition from top5 to top25. This result highlights the capability of the TripleTOF 6600 System for fast MS/MS acquisition, which allows for the fragmentation of a large number of precursors in a single DDA cycle, leading to a larger number of metabolites identified.
Figure 3. Evaluating the DDA strategy for untargeted metabolomics workflows. Gain in metabolite coverage in a plasma extract using various DDA acquisition methods with constant acquisition time above the Top5 DDA method. There is over 100% increase of metabolite coverage in plasma extracts by increasing the number of selected precursor ions for DDA acquisition from top5 to top25.
SWATH Acquisition
In the second part of this study, the SWATH Acquisition strategy was tested with various fixed (fw) and variable window (vw) sizes with similar cycle time in a plasma extract. As shown in Figure 4, increasing the number of fixed windows resulted in ~30% gain in metabolite coverage. Using the variable window method resulted in a ~70% gain in metabolite coverage.
These results show that decreasing the window size and varying the window size, depending on the precursor ion mass density, improves the overall ion selectivity as shown in Figure 5. This highlights an example of the MS/MS spectrum of D-Lysine with a precursor mass 147.1125 m/z. Here one can visualize a Q1 window from 127.1-149.4 m/z (top) and Q1 window from 137.7-149.4 m/z (bottom) acquired using a SWATH Acquisition method with 15 variable windows and 30 variable windows, respectively. Figure 5 (top panel) clearly show many fragments from other co-eluting metabolites infiltrating the MS/MS spectrum within the mass range from 127.1 m/z to 149.4 m/z. This highlights the need for a higher number of SWATH Acquisition windows with narrower widths, allowing fewer precursor ions selected for MS/MS fragmentation, resulting in higher specificity and selectivity, necessary for confident metabolite identification.
Figure 4. Evaluating the DIA strategy for untargeted metabolomics workflows. Gain in metabolite coverage in a plasma extract using various SWATH Acquisition approaches with constant cycle time. Increasing the number of windows selected for SWATH Acquisition resulted in ~30% gain in metabolite coverage using fixed windows (blue) and ~70% gain in plasma extracts using the variable windows (red) strategy.
Figure 5. The SWATH Acquisition variable windows effect on fragmentation spectra in plasma extract. Fragmentation spectra for a metabolite, D-Lysine highlighting the comparison between 15 (top) and 30 (bottom) variable windows with a cycle time ~0.6 seconds in plasma extract. The blue spectrum represents all measured MS/MS ions and the grey spectrum shows the library spectrum of D-Lysine from the Accurate Mass Metabolite Spectral Library. It can be observed that a higher number of Q1 windows allows for cleaner data and hence leads to a higher specificity resulting in a higher quality spectral match to the library.
Comparing identification rates
In the third step, identification rate was compared between SWATH Acquisition and traditional DDA acquisition in a plasma extract. At first glance DDA acquisition presents a higher number of metabolites identified solely based at the MS1 level (Figure 4, MS acquired); however when the MS/MS is used to confirm the metabolites, the numbers of identified metabolites drops significantly; most likely due to the sheer number of false positives using just the MS1 data (and mass accuracy alone). These numbers drop further when the library score is set to 70% and above meaning that the MS1, MS/MS, isotope distribution and retention time must have a combined scoring of 70% and above to be considered a high level identification (Figure 6).
The SWATH Acquisition method is using information obtained from both the MS and MS/MS spectrum. Due to this, metabolites are identified not only on their exact mass, but also based on their molecular structure. Figure 5 also shows an increased number of metabolites identified at the MS/MS level for samples measured using SWATH Acquisition.
Finally, these experimental approaches were applied to common matrices used in metabolomics studies, namely urine and extracted plasma. Figure 6 illustrates that SWATH Acquisition applying 20 variable windows can identify up to 55% more metabolites than a traditional top20 DDA acquisition (in a urine extract). More confident MS/MS based identifications lead to higher quantifiable metabolites in a metabolite expression experiment, which at the end allows better understanding of the biology. When comparing the performance in extracted plasma it can be observed that applying SWATH Acquisition with 20 variable windows allows significant gains in metabolite coverage (around 55%) versus the top20 DDA acquisition, similar gains as seen in the urine extract (Figure 7),
Lastly note that the larger the library, the more coverage one can gain from a sample. The library used for these experiments even though small in size, contained high quality spectra from biochemically relevant metabolites generated on TripleTOF Systems.
Figure 6. Increase in metabolites identified with MS/MS-based acquisition strategies in plasma extract. Number of metabolites identified based on MS1 level with and without MS/MS confirmation using DDA (top25) and SWATH Acquisition (fw30 and vw30). It is observed that a larger number of metabolites are identified based on MS1 only, but the number of identifications drops when using MS/MS criteria highlighting the large number of false positives using MS1 only based identification. When comparing the DDA method with the SWATH Acquisition approaches one can see the increase in number of metabolites identified (MS2 Acquired). Applying library matching criteria of scores above 70% mean a higher quality metabolite identification with vw30 resulting is a larger number of identifications vs fw30 and DDA top25 approaches.
Figure 7. Gain in metabolite coverage in urine and plasma extracts. (Top) 55% gain in metabolite coverage with top20 DDA and vw20 SWATH Acquisition in human urine. Graph is normalized to the highest library coverage, SWATH Acquisition with 20 variable windows. (Bottom) Over 55% gain in metabolite coverage with top20 DDA and vw20 SWATH Acquisition in human plasma. Graph is normalized to the highest library coverage, SWATH Acquisition with 20 variable windows. For comparison, methods with the same MS/MS accumulation of 25 ms were used.
Conclusions
- Over 50% gain in metabolite coverage and greater MS/MS level metabolite identification was shown using the Accurate Mass Metabolite Spectral Library when employing variable window SWATH Acquisition compare to traditional DDA acquisition.
- Using variable windows instead of fixed windows significantly improved the metabolite coverage.
- Increasing the number of windows will refine the quality of the MS/MS spectra, thereby increasing the selectivity, and yield higher quality metabolite identifications.
- Setting up the correct Q1 window width can be curial when measuring different matrixes.
- Furthermore, the advantages of SWATH Acquisition allow no method development by employing a generic method setup. Generate a digitized map of your samples metabolome and never re-run a sample ever again, just re-mine the data collected!
References
- Gillet LC et al (2012) Mol. Cell. Prot. 11(6), 1-17.
- Tsugawa H et. al., (2105) Nat. Methods. 12(6), 523-6.
- Improved data quality using variable Q1 window widths in SWATH® Acquisition. SCIEX technical note RUO-MKT-02-2879-B.
- Download the SWATH Variable Window Calculator - Excel tool.
Related content
- Improved metabolite identification using data independent analysis for metabolomics. SCIEX technical note RUO-MKT-02-10617-A.
 Click to enlarge
Click to enlarge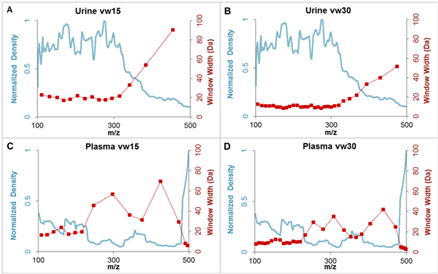 Click to enlarge
Click to enlarge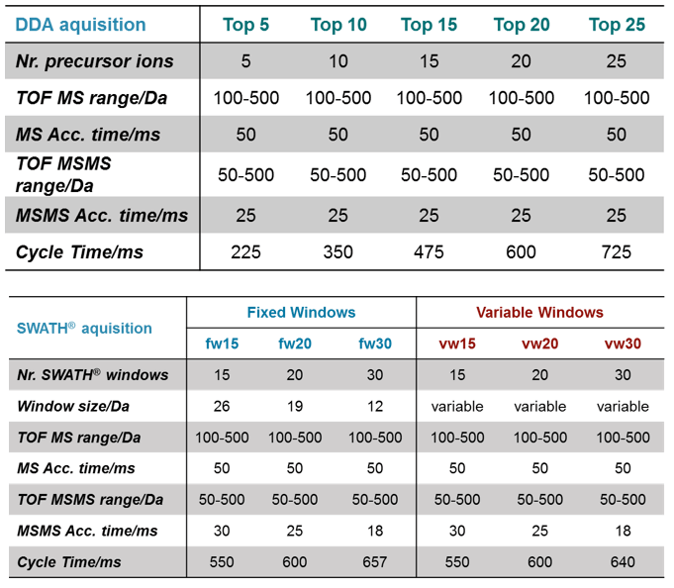 Click to enlarge
Click to enlarge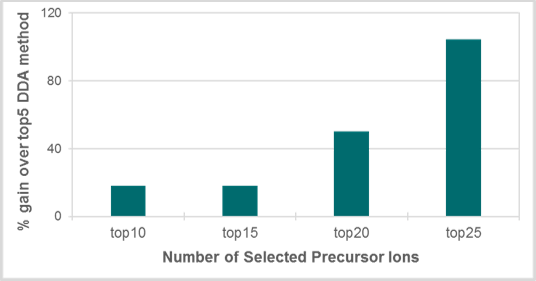 Click to enlarge
Click to enlarge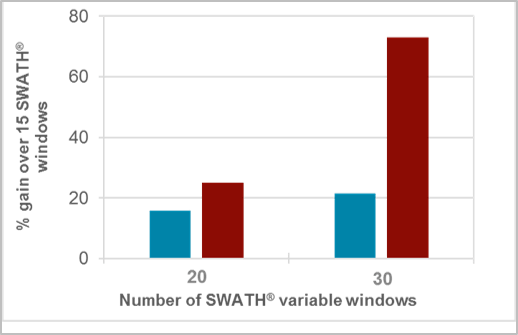 Click to enlarge
Click to enlarge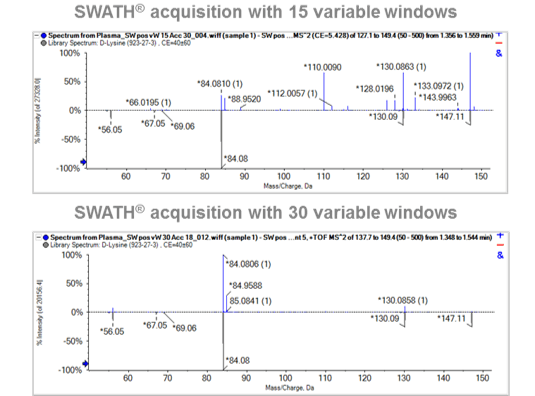 Click to enlarge
Click to enlarge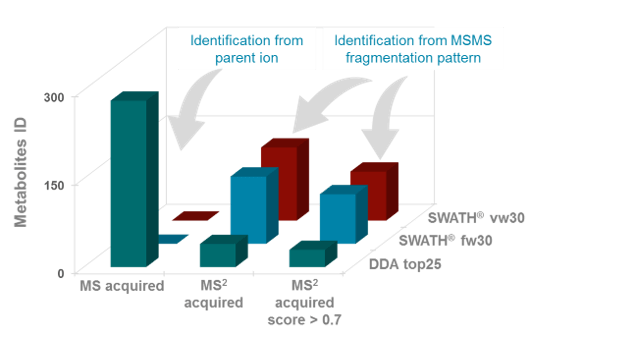 Click to enlarge
Click to enlarge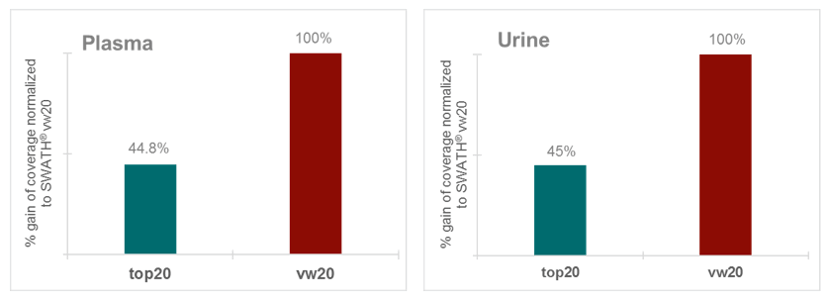 Click to enlarge
Click to enlarge