Achieving very high reproducibility for quantitative proteomics with nanoflow and microflow LC-MS
NanoLC™ 400 Series System
Christie Hunter
SCIEX, USA
Introduction
From simple protein identification experiments to global protein expression workflows, proteomic applications require reproducible, reliable chromatography. The reliability, ease of use and simplicity of design has made the NanoLC 400 Systems the market leader in low flow separations.
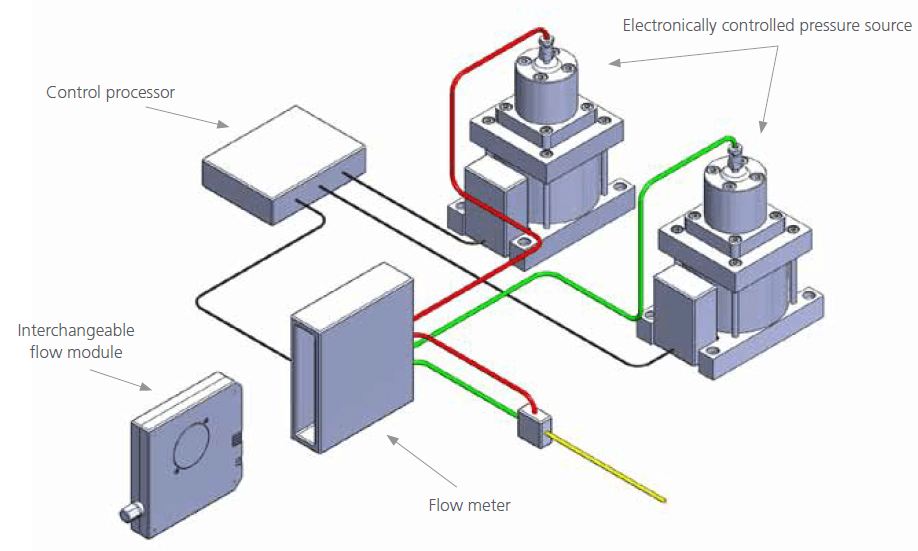
The NanoLC 400 System employs a novel Microfluidic Flow Control™ Technology (MFCPlus™) for direct pumping of solvent flow (Figure 1). This direct pumping system eliminates the need for flow splitting, alleviating the flow inaccuracies of splitter based systems and reducing solvent consumption and waste. The system uses flow meters to monitor the flow rate in each mobile phase channel with extreme precision (<1 nL/min). Flow rate information is continuously fed to a microfluidic flow controller which rapidly adjusts a variable pressure source to maintain constant flow. Each mobile phase is monitored independently before mixing, providing precise gradient formation in addition to excellent flow stability that is unaffected by downstream pressure fluctuations.
The NanoLC 400 System with MFCPlus™ Technology provides excellent reliability, reproducibility and flexibility.
Figure 1. Microfluidic Flow Control™ Technology (MFCPlus™) in the NanoLC 400 System. Solvent flow in each channel is monitored with extreme precision and is maintained by constant feedback to the pressure source. This enables flow rate stability and gradient precision across the LC gradient. Switching the interchangeable flow module allows the user to rapidly switch between flow rate ranges.
Key features of the NanoLC 400 System for quantitative proteomics
- The NanoLC 400 System has the flexibility and reliability to support a broad range of workflows, from global discovery to targeted quantitation
- Switching the interchangeable flow module allows the user to rapidly switch between flow rate ranges
- Highest reproducibility in retention time for targeted quantitative workflows
- MFCPlus™ Technology provides flow stability for highest retention time reproducibility, with retention time variability below 0.35% RSD at 500 nL/min
- High pressure LC system (10 000 psi)
- High precision autosampler enables high injection reproducibility with little or no sample waste.
Retention time and peak area reproducibility
Quantitative LC-MS applications, such as biomarker discovery by MS profiling or targeted peptide quantitation using the Scheduled MRM™ Algorithm, require the comparison of chromatographic peak areas across many different samples. These applications demand high LC reproducibility allowing narrow peak detection windows to be used for analysis.
Retention time reproducibility at nanoflow rates was measured for tryptic peptides in an E. coli cell lysate digest (Figure 2). Excellent reproducibility was achieved across 25 replicate injections and the average retention time variability was 0.21%. Reproducibility at microflow rates was also assessed, using a set of standard peptides (Figure 3, top left). Retention time RSD of 0.18% was achieved here as well, demonstrating excellent stability across a range of flow rates. Peak area reproducibility was also extremely high for the micro flow experiment, showing constant signal and low %CVs across 50 injections (Figure 3).
Figure 2. Retention time reproducibility at nanoflow rates. Seventy peptides from an E.coli cell lysate tryptic digest were separated using a 75 µm ID column at 300 nL/min and monitored using Scheduled MRM™ Algorithm on the QTRAP® 5500 System (top). 25 replicate injections were performed and the retention time of each peptide is plotted (bottom). The average retention time RSD was 0.21%.
Figure 3. Retention time and peak area reproducibility at microflow rates. 50 replicate injections were performed monitoring a 21 peptide standard mix using a 300 µm ID ChromXP™ column at 4 µL/min with a 10 minute gradient. (Top left) TIC overlays for injections 10, 20, 30, 40 and 50 highlight the very high reproducibility. (Top right) Peak area %CV for each MRM across the 50 injections was computed and plotted vs average peak area, the average peak area %CV across all MRMs was 5.1% CV. Peak area reproducibility was very high with minimal drift across the dataset (bottom, plotted in log scale to show full dynamic range).
Sample injection reproducibility
In addition to high retention time reproducibility, reproducible sample injections are a key component of quantitative proteomic applications. The NanoLC 400 autosampler has been engineered to provide fast, high precision sample injection. For many proteomic applications where minimal sample is available, the µL-pickup injection method can be used to minimize sample consumption. This injection method, combined with automatic vial-bottom sensing means small injection amounts can be made reproducibly from low volumes of precious samples, typically in less than three minutes. A series of injection volumes were performed using µL-pickup in a 10 µL sample loop and the peak areas were monitored using UV detection (Figure 4). Excellent linearity and reproducibility were observed from 0.2 – 7 µL injection volumes.
Figure 4. Sample injection reproducibility using the µL-pickup injection mode. Five or more replicate injections across a range of sample volumes were injected and peak areas were measured using UV detection. Excellent linearity was achieved and the average %CV of the peak areas was <1.5%. |
Quantitative proteomics
High retention time reproducibility and sample injection precision are critical acquisition attributes when performing comprehensive quantitative applications such as SWATH® Acquisition. Replicate analysis of a digested yeast cell lysate sample was performed using the NanoLC 400 System on a TripleTOF® System with SWATH Acquisition to assess whole system reproducibility (Figure 5). In this application, sample injection, retention time and MS reproducibility are key performance factors for highest quality data. Retention time stability is critical as fragment ions from specific peptides are extracted from LC-MS datasets using narrow retention time windows to ensure the correct peptides are detected and quantified across every sample.
Figure 5. High workflow reproducibility for SWATH Acquisition applications. Five replicate injections of yeast cell lysate digest were analyzed on the TripleTOF® 5600 System using the SWATH Acquisition workflow. The total ion chromatograms for the five experiments overlay very well (top) highlighting the high LC-MS reproducibility of the whole system. Peak areas for fragment ions from ~3500 yeast tryptic peptides were extracted across the 5 experiments and the reproducibility of the data at the transition, peptide and protein level is shown (bottom). At the fragment / transition level, almost 80% of the data has reproducibility better than 20% CV.
Conclusions
The flexibility and reproducibility of the NanoLC™ 400 System makes it an excellent LC system for labs performing proteomics workflows, from protein expression analysis to high throughput peptide quantitation.
- Retention time reproducibility of 0.2% RSD was routinely observed at both micro and nanoflow regimes.
- The fast, high precision autosampler provided accurate, reproducible sample injections (<1.5% CV) even in µL-pickup mode such that peak area reproducibility across quantitative workflows was very good.
- High injection precision resulted in very good peak area reproducibility in both the MRM and SWATH® Acquisition experiments, ensuring quantitative robustness is easily achievable for targeted quantitative proteomics.
References
- Exploring the sensitivity differences for peptide quantification in the low flow rate regime - NanoLC™ 400 System for high performance nanoflow and microflow LC, SCIEX technical note RUO-MKT-02-3252-A.

 Click to enlarge
Click to enlarge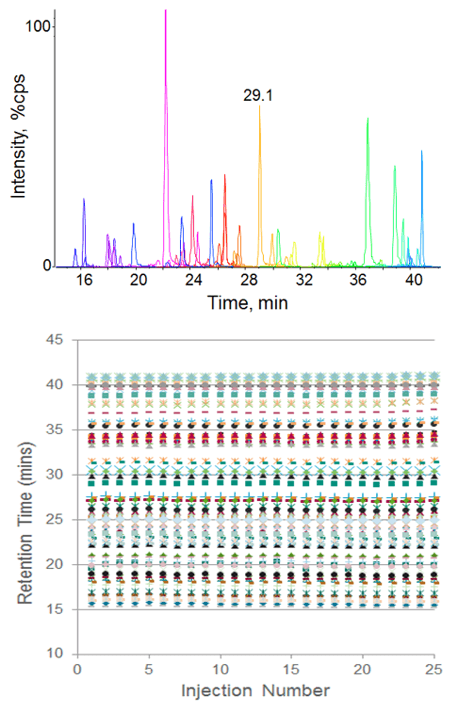 Click to enlarge
Click to enlarge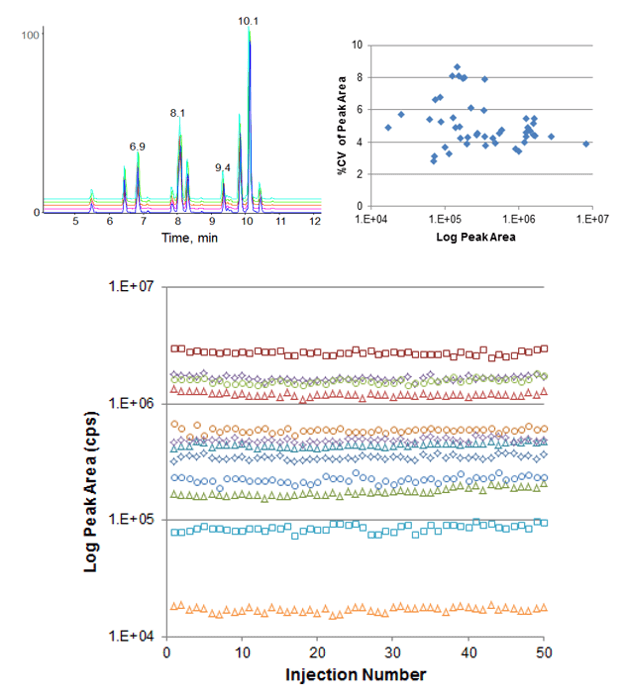 Click to enlarge
Click to enlarge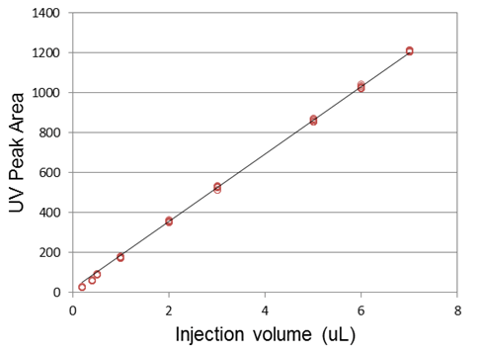 Click to enlarge
Click to enlarge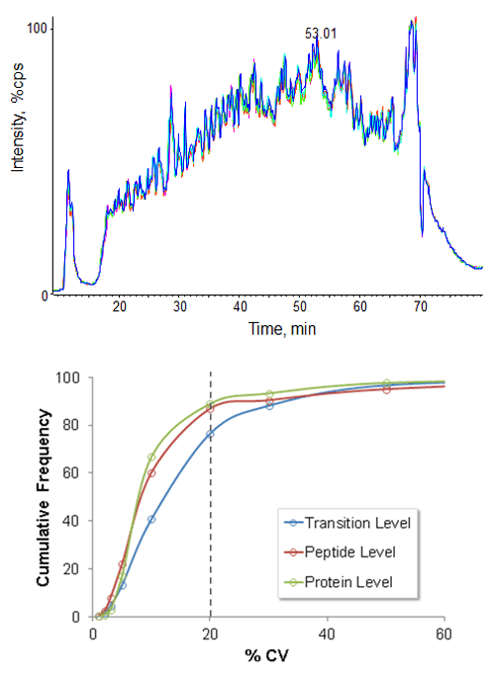 Click to enlarge
Click to enlarge