How to ensure compliance for your biotherapeutics from development throughout quality control (QC)
Featuring SCIEX OS Software
Giulia Calloni1 , Sibylle Heidelberger2 , Kerstin Pohl3
1SCIEX, Germany; 2SCIEX, UK; 3SCIEX, USA
Abstract
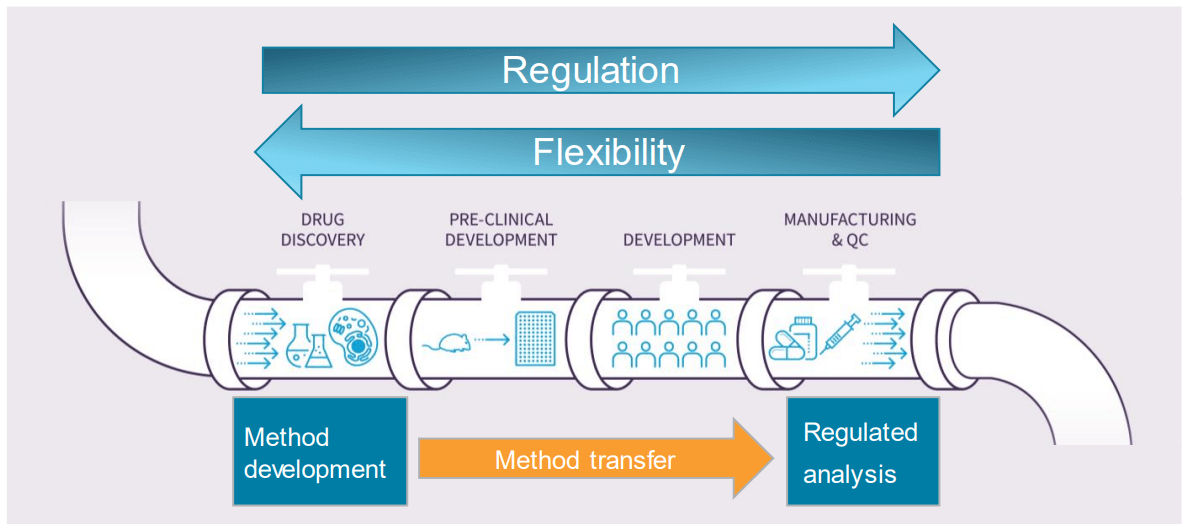
Mass spectrometry (MS) is increasingly used as an analytical method for the characterization and quantification of biotherapeutics across the entire production pipeline (Figure 1), from early development stages to the final production. This approach provides information-rich, specific results. It allows both specific countermeasures and a reduction in time and resources. Being used at different stages of the biotherapeutics life cycle, MS acquisition and analysis systems have to cope with very different requirements (Figure 1). While in the discovery and early development phases, freedom and flexibility are needed. During later stages, as in quality assurance and control (QA/QC), ease of use and the fulfillment of regulatory requirements (above all) are mandatory. In addition, analytical methods that are developed during early phases, without validation and regulations, need to be transferred to the later stages for routine analysis in a regulated environment. SCIEX OS Software is a GxP compliant-ready solution that integrates data acquisition and processing allowing for flexibility while meeting compliance requirements.
Figure 1. At different stages during the life cycle of a biotherapeutic, the analytical systems must fulfill different requirements. Moving downstream from drug discovery and early development to manufacturing and QC, the methods developed initially without regulations must be made routine and carried out in a strictly regulated manner.
Key features of SCIEX OS Software
- A single, easy-to-use software for streamlining workflows through method development, data acquisition and the analysis of qualitative and quantitative data—eliminating the need for learning different software packages
- GxP compliant-ready system with great flexibility in defining customized user roles, adjusting to the specific needs for each individual laboratory
- Easy to set up and customize audit trail functionalities assuring data integrity and traceability, with the possibility to create compliant-based projects and/or workstation
- Fully customizable workflows for characterization and quantification of the biotherapeutics in a compliant environment, including intact, subunit and peptide-based MAM workflows, as well as intact, subunit and peptide-based quantification workflows
 Click to enlarge
Click to enlarge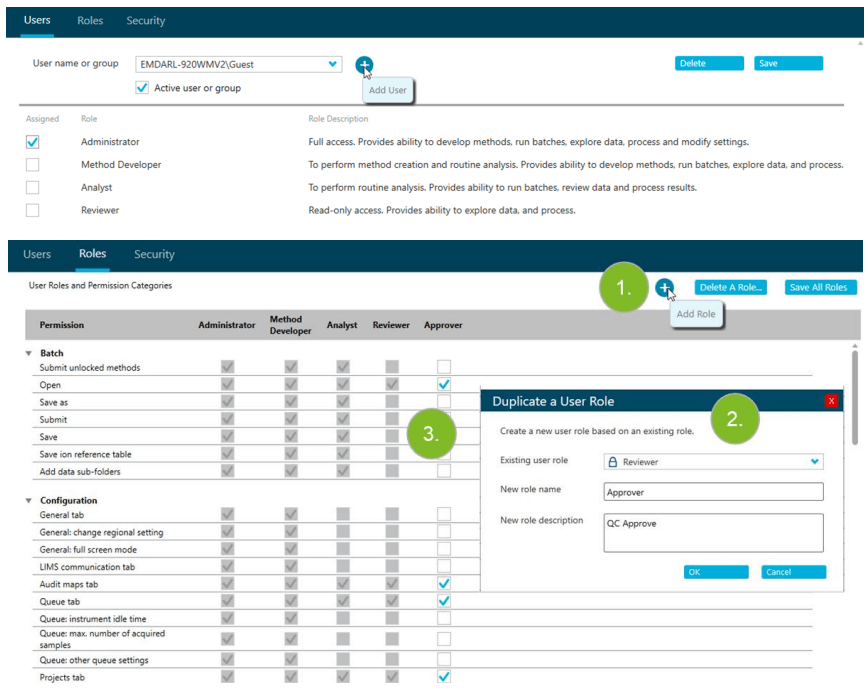 Click to enlarge
Click to enlarge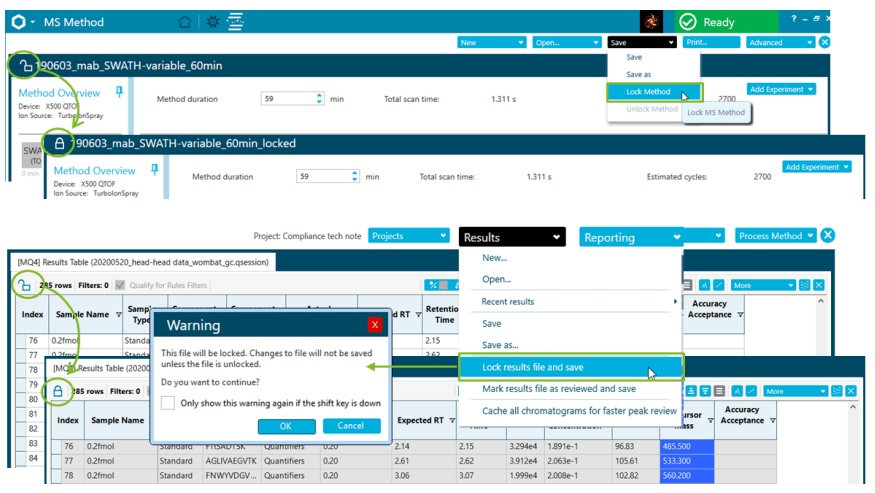 Click to enlarge
Click to enlarge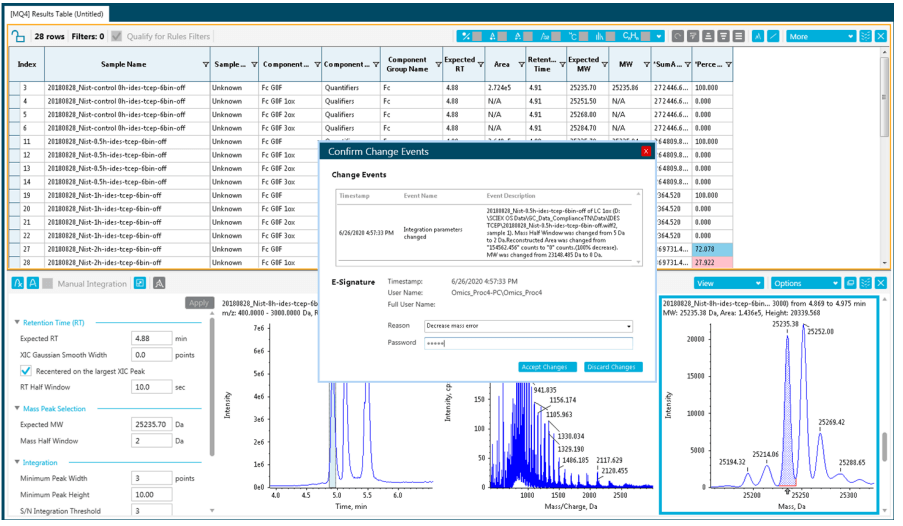 Click to enlarge
Click to enlarge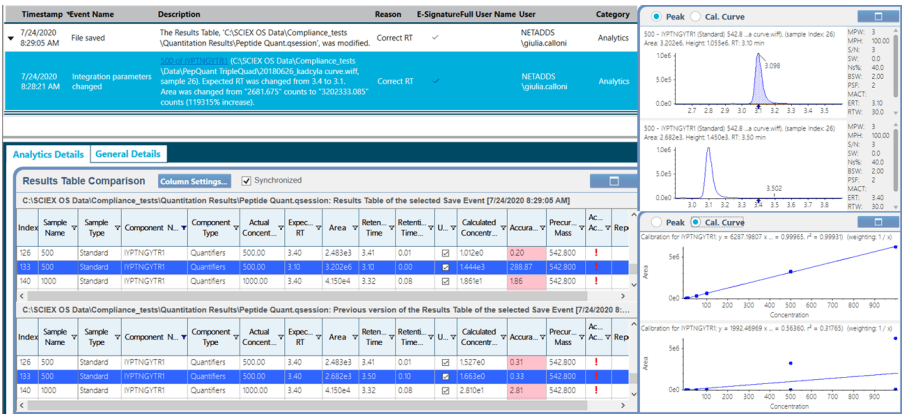 Click to enlarge
Click to enlarge