Intermediate precision study of the CE-SDS assay on the BioPhase 8800 system with native fluorescence detection
Abstract
In the biopharmaceutical industry, capillary electrophoresis–sodium dodecyl sulfate (CE-SDS) assays are routinely utilized to evaluate critical quality attributes (CQAs) of many protein therapeutics. While ultraviolet (UV) absorbance and laser-induced fluorescence (LIF) are commonly used detection methods, they do have some inherent limitations, such as baseline noise interference to low-level impurity detection with UV and the need for time-consuming labeling with LIF.
To overcome these limitations, SCIEX has incorporated native fluorescence detection (NFD) on the BioPhase 8800 system to offer label-free protein CQA analysis with a sensitivity higher than UV detection. This technical note highlights results from an intermediate precision study demonstrating excellent intra-capillary and inter-capillary reproducibility for both relative migration time and corrected peak area percentage, as well as improved sensitivity in detecting low-level impurities compared to UV detection.
Key features of CE-SDS with NFD
- Time-efficient data processing: Faster, more confident peak integration than with traditional UV due to significantly reduced noise and flattened baseline
- Enhanced detection sensitivity: The enhanced sensitivity of NFD enables improved detection and quantitation of low-abundant species compared to UV detection
- Excellent intra- and inter-capillary reproducibility: In intra-capillary testing, %RSD was < 0.1% for RMT and < 0.4% for CPA% of the heavy chain. In inter capillary testing, %RSD was < 0.1% for RMT and < 0.3% for CPA% of the heavy chain.
- Kit-based workflows: Complete CE-SDS kit and pre-assembled bare fused silica cartridge facilitate consistency and overall data reproducibility
Introduction
Protein therapeutics—such as monoclonal antibodies (mAbs), bispecifics, and fusion proteins—are a well-established class of drugs in the biopharmaceutical industry. To ensure drug safety and efficacy, multiple CQAs such as purity, fragmentation, aggregation, and post-translational modification need to be well characterized and monitored. The CE-SDS assay offers automated and quantitative quality assessments of protein therapeutics and is routinely used as a product release test. UV absorbance is the most widely used detection method for CE-SDS, with protein variant levels typically reported as corrected peak area percentage (CPA%). However, UV absorbance by non-analytes in the capillary and heterogeneity in gel buffer can lead to baseline fluctuations, complicating peak integration and slowing down data analysis. Additionally, UV detection requires a high sample concentration. Although the sensitivity of the CE-SDS assay can be increased using LIF detection, the protein samples need to be labeled with a fluorescent dye. The labeling process is often time-consuming and may introduce sample preparation artifacts, as well as changes in the physical properties of the molecule.1
NFD provides a label-free alternative by utilizing the intrinsic fluorescence of aromatic amino acids—primarily tryptophan, with contributions from tyrosine and phenylalanine to a lesser extent. Tryptophan residues exhibit strong absorption at 280 nm and emit fluorescence near 350 nm upon excitation. This natural property enables sensitive detection without chemical derivatization, providing a stable, flattened baseline that facilitates easier peak integration and faster data analysis compared to UV detection.
The BioPhase 8800 system provides processing of up to 8 samples in parallel for the quality assessments of protein and nucleic acid therapeutics as well as viral vectors for gene therapy. The addition of NFD to the BioPhase 8800 system expands the UV and LIF detection workflows to also include CE-SDS-NFD, CZE-NFD, CGE-NFD, and cIEF-NFD.
This technical note discusses the enhanced performance of NFD, featuring improved sensitivity, a more stable baseline, and easier peak integration compared to UV detection. In addition, the results of an intermediate precision study using CE-SDS on the BioPhase 8800 system with NF are presented.
Methods
Materials: The BioPhase CE-SDS Protein Analysis Kit (P/N: C30085), the BioPhase BFS capillary cartridge - 8 x 30 cm (P/N: 5080121), and the BioPhase sample and reagent plates (4,4,8) (P/N: 5080311) were from SCIEX (Marlborough, MA). The β-mercaptoethanol (β-ME) (P/N: M3148-25ML), Iodoacetamide (IAM, P/N: I-1149), and Lysozyme (10 mg/mL, P/N: L3790-10 x 1 mL) were from MilliporeSigma (St. Louis, MO). NISTmAb, Humanized IgG1k Monoclonal Antibody (P/N: 8671) was from NIST (Gaithersburg, MD).
Preparation of reduced IgG control samples: A 950 μL of IgG control standard was combined with 50 μL of β-ME and 100 μL of 10 kDa internal standard. The sample mixture was vortexed, briefly centrifuged at 2,500 g, then heat denatured at 70°C for 10 min. Afterward, the sample was allowed to cool to room temperature. The samples were then pooled and redistributed to avoid sample-to-sample variation. One column for NFD, one column for UV 220 nm). 100 μL of the sample solution was transferred to each well of the sample plate for CE-SDS analysis with 6 injections per well on the BioPhase 8800 system with NF.
Preparation of lysozyme stock solution: The SDS sample buffer at a volume of 990 μL was transferred to a 1.5-mL microcentrifuge tube. Then, 10 μL of Lysozyme (10mg/mL) was added. The tube was capped and mixed thoroughly using a vortex mixer, followed by a quick spin.
Preparation of the IAM alkylating agent working solution: 46 mg of IAM was added to a 1.5 mL microcentrifuge tube. The IAM was dissolved by adding 1 mL of double-deionized (DDI) water. The tube was capped and mixed thoroughly using a vortex mixer. After IAM was completely dissolved, the solution was consolidated by a quick spin and then stored in the dark at room temperature. The solution is stable for approximately 24 hours at room temperature.
Preparation of non-reduced NIST IgG with lysozyme: Four microcentrifuge tubes were labelled with “0”, “0.1%”, “0.2%”, and “0.4%”, and 720, 712, 704, and 688 μL of CE-SDS sample buffer were added to them, respectively. Then, 40 μL of IAM working solution and 80 μL of NIST IgG1k (10 mg/mL) were added to each tube. Next, 0, 8, 16, and 32 μL of the lysozyme stock solution prepared earlier were added to tubes labelled with “0”, “0.1%”, “0.2%”, and “0.4%”, respectively. Tubes were capped tightly, sealed with Parafilm, and mixed thoroughly using a vortex mixer, followed by a quick spin. The tubes were heated in a water bath at 70 °C for 10 minutes. After a quick spin, the tubes were placed at room temperature for at least 3 minutes. 100 μL of the sample solution was transferred to each well of the sample plate for CE-SDS analysis, with 3 injections per well on the BioPhase 8800 system using NFD.
Preparation of non-reduced USP IgG: In amicrocentrifuge tube, 720 μL of CE-SDS sample buffer was mixed with 40 μL of IAM working solution and 80 μL of the USP IgG (10 mg/mL). The tube was capped tightly, sealed with Parafilm, and mixed thoroughly using a vortex mixer, followed by a quick spin. The tubes were heated in a water bath at 70 °C for 10 minutes. After a quick spin, the tubes were placed at room temperature for at least 3 minutes. 100 μL of the sample solution was transferred to each well of the sample plate for CE-SDS analysis with 3 injections per well on the BioPhase 8800 system with NF and UV detection in the same sequence run.
Instrumentation: The BioPhase 8800 system, equipped with an NF detector with a 350 nm filter (P/N: 5314860), was from SCIEX. The Multi-Therm shaker incubator (P/N: H5000-H) was from Benchmark Scientific (Sayreville, NJ).
Software: The BioPhase software package version 1.5 was used for creating run methods and sequences, and for data acquisition and processing.
Methods: The capillary conditioning, separation, and shutdown methods were performed as described in the CE-SDS kit for the BioPhase 8800 system application guide.2 The temperature of the sample storage compartment and separation cartridge was always set at 25°C.
Results and discussion
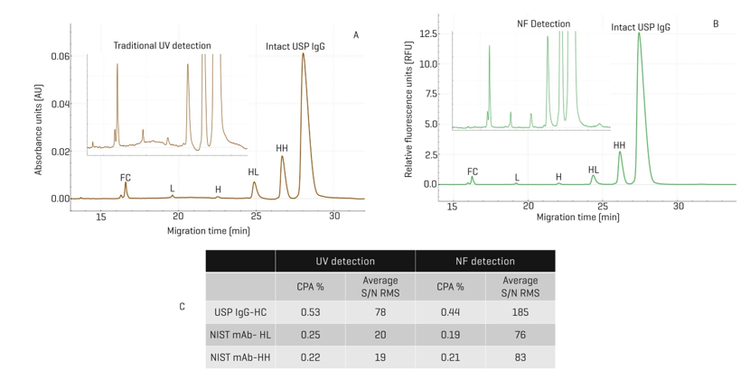
Enhanced quantitation of protein fragments with NFD relative to UV detection: In Figure 1, results from CE-SDS analysis of non-reduced USP IgG with NFD were compared with those with UV detection. In panel A, the electropherogram obtained with UV detection displayed the intact USP IgG peaks as well as various fragments: HH, HL, H, L, and FC. The zoomed-in view in the inset shows the fluctuation of the baseline, complicating the peak integration. In contrast, the baseline with NFD is clearly stable and flat, making peak integration easier and with higher confidence in rhe results. Panel C summarizes the CPA% and the average S/N ratio RMS for representative fragments from 2 experiments, one with USP IgG and another with NIST mAb, using both UV detection and NFD. With the USP IgG, the abundance of the HC fragment was similar with UV and NFD (0.53% vs. 0.44%). However, the S/N RMS obtained with NFD was 185, significantly higher than the 78 obtained with UV detection. Similarly, the CPA% values for the NIST mAb-HL and NIST mAb-HH fragments were 0.25% and 0.22% respectively, close to the 0.19% and 0.21% obtained with the NFD. Here, again, the average S/N RMS values obtained with NFD were 76 and 83 for NIST mAb-HL and NIST mAb-HH fragments, significantly higher than the 20 and 19 obtained with UV detection. In summary, these results demonstrate a better sensitivity with a higher S/N ratio, a more stable baseline, and easier peak integration with NFD than with UV detection in the quantitation of protein fragments with NFD in comparison with UV detection.
Design of the intermediate precision study: Figure 3 shows the intermediate precision study design scheme. The approach used in this study sought to determine the impact of the instrument, operator, capillary cartridge, and chemistry kit lot on inter- and intra-capillary reproducibility. The study took only 2 days of laboratory and instrument hands-on work and 1 day for data analysis and reporting.
This design assessed the assay and instrument performance using a reduced IgG control standard. Several parameters were monitored to determine the intra- and inter-capillary reproducibility, including: 1) CPA% and relative migration time (RMT) of low molecular weight impurity (LMW), light chain (LC), non-glycosylated heavy chain (ng-HC), and heavy chain (HC); 2) CPA% ratio between HC and LC, and 3) the resolution between ng-HC and HC.
Intra and inter-capillary reproducibility using IgG control standard: A total of 48 injections of the reduced IgG control standard were performed by each operator on a representative instrument. Figures 1A and 1B display the stacked electropherograms of the IgG control standard, respectively. In Figure 4A, 6 traces obtained from 6 injections in the same capillary showed identical profiles with LMW, LC, ng-HC, and HC peaks clearly identified, demonstrating excellent intra-capillary reproducibility and injection consistency. Figure 4B shows stacked electropherograms obtained with 1 injection per capillary. The profiles obtained in all 8 capillaries from A to H were very consistent, demonstrating outstanding inter-capillary reproducibility.
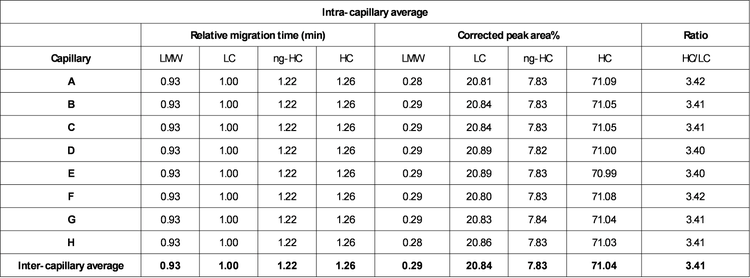
Relative migration time: Figures 4A and 4B show a good overlay of the LC peak that was used to calculate the relative migration times (RMT) of LMW, ng-HC, and HC. Table 1 summarizes the intra- and inter-capillary average values for RMT. Overall, the average values (N=144) for RMT were consistent inter- and intra-capillaries, indicating the assay's specificity, reproducibility, and the robustness of the cartridge cooling features of the BioPhase 8800 system. Table 2 highlights a %RSD better than 0.08% for the RMT for all species of reduced IgG control standard.
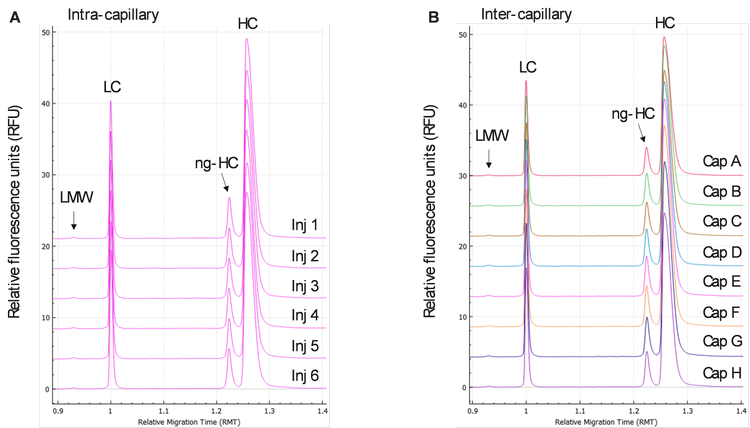
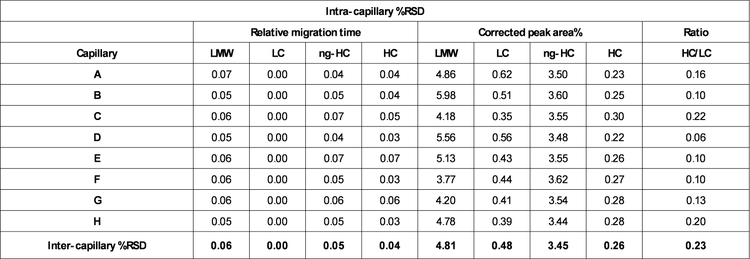
Corrected peak area%: The CPA% values were determined automatically by the BioPhase software. Briefly, the CPA% was calculated by dividing the peak area by the migration velocity of the corresponding peak. The average values for the CPA% of the 4 species found in the reduced IgG control standards (LMW, LC, ng-HC, and HC) are displayed in Table 1. Consistent values for CPA% were observed for the reduced form of the IgG control standards across all 8 capillaries. Moreover, this result was reproducible across the 3 systems tested, highlighting inter- and intra-capillary reproducibility. The overall intra-capillary %RSD for CPA% for the LC and HC was between 0.22% and 0.62%. For low-level impurities, LWM and ng-HC, the overall intra-capillary %RSD was below 6.00%, indicating excellent intra-capillary reproducibility and assay precision.
HC/LC ratio: The ratio between the CPA% for the HC and LC was automatically calculated by the software without any additional user input. The average intra-capillary values are shown in Table 1 and indicate data consistency. A %RSD of the HC/LC ratio between 0.10% and 0.22% was observed intra-capillary and 0.23% for inter-capillary, indicating excellent reproducibility (Table 2).
Resolution ng-HC and HC: Another critical quality attribute is the resolution between the ng-HC and HC. The presence of a ng-HC peak is considered a product-related impurity because it alters the stability and effector functions of the final therapeutic product. Therefore, it is essential to separate and quantify this variant consistently.3 The mobility of these species is very similar, and therefore, it is necessary to monitor resolution and specificity during the CE-SDS separation under reduced conditions. Figure 5 shows the resolution obtained across 3 operators (N=144 injections). The overlay of the data points corresponding to the data from the 3 instruments suggests consistent baseline resolution between ng-HC and HC across capillaries, cartridges, and instruments. The overall reproducibility achieved for the resolution between the ng-HC and HC resolution is better than 0.70 %RSD.
High assay sensitivity with CE-SDS-NFD: In this experiment,the lysozyme was spiked with USP IgG at 0, 0.1%, 0.2% or 0.4%. Each sample was injected 3 times. The left panel displays a stacked view of representative electropherograms obtained at different lysozyme concentrations. No lysozyme peak was detected in the trace obtained with the sample that had no lysozyme spiked. However, in 3 other traces obtained with the lysozyme spiked into the IgG sample, the lysozyme peak was detected in addition to the intact IgG and its fragments. In all 4 traces, a stable and flat baseline was observed. The inset shows a zoomed-in view of the overlay of the 4 traces demonstrating a dose-dependent increase of the lysozyme signal. The right panel shows a linear plot of the average S/N RMS against the concentration of the spiked-in lysozyme. The error bar shows the standard error of the mean. The R2 value of the linear plot was 0.9999, indicating high accuracy of the assay. A formula was generated from the plot. Using this formula, the S/N RMS value was extrapolated to be 15, demonstrating a sensitivity better than 0.01% in detecting impurities. The S/N RMS obtained at 0.1% spiked-in lysozyme was 156 with NFD, which is significantly higher than 30 obtained with the UV detection at 220 nm. This indicates a 4-fold increase in sensitivity.
Conclusion
- A stable baseline was observed with NFD, enabling easy peak integration and faster analysis time
- Better S/N ratio and sensitivity were obtained with NFD in comparison to UV detection, enabling confident detection of low-level impurities
- Excellent inter- and intra-capillary reproducibility for RMT and CPA% for LMW, LC, ng-HC, and HC between all parameters studied, illustrating robustness and consistency across the BioPhase 8800 system with NF, cartridges, and chemistry kits
- The combination of a complete chemistry kit and pre-assembled cartridge facilitated the throughput and robustness of this study
- The BioPhase 8800 system enables the user to acquire data using both UV and NF detection schemes in the same sequence without interrupting the workflow
References
- Oscar Salas-Solano, Brandon Tomlinson, Sarah Du, Monica Parker, Alex Strahan, and Stacey Ma, Optimization and Validation of a Quantitative Capillary Electrophoresis Sodium Dodecyl Sulfate Method for Quality Control and Stability Monitoring of Monoclonal Antibodies; Anal. Chem. 2006, 78, 6583-6594.
- BioPhase CE-SDS Protein Analysis kit application guide.
- Kai Zheng, Christopher Bantog, and Robert Bayer; The impact of glycosylation on monoclonal antibody conformation and stability; MAbs. 2011 Nov-Dec; 3(6): 568–576.

