Abstract
This technical note highlights an AOAC official method developed by Nestlé for the screening of 152 veterinary drug residues in food-producing animals by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The method, developed on SCIEX instruments, was recognized as AOAC Official Method 2020.04.1 It provides a comprehensive platform capable of detecting veterinary drugs across a variety of food matrices, including milk-based products, meat- and fish-based products, eggs-based products, animal fat and animal byproducts.
Key benefits for the analysis of veterinary drugs in food using SCIEX LC-MS/MS technology
- Comprehensive detection: The method screened for 152 veterinary drug residues across a diverse range of food products, making it one of the most extensive multi-residue detection platforms available (Figure 1)
- High sensitivity and accuracy: The method is capable of detecting residues at very low concentrations, at or below the maximum residue limits (MRLs) established by regulatory bodies
- Method validation: Multi-laboratory validation led to probability of detections (PODs) at the screening target concentration levels (STC) ≥94% and PODs in the blank ≤9%
- Official AOAC method: The method was elected as AOAC method of the year in 2022 and was established the AOAC Final Action Official Method 2020.04
Introduction
Veterinary drug residues are substances that can remain in animal products such as meat, dairy, eggs, and fish when these drugs are used to treat, prevent, or manage diseases in food-producing animals. These residues are of concern due to their potential to cause adverse effects on human health, including antimicrobial resistance (AMR), allergic reactions, and even carcinogenic or teratogenic outcomes.2 In light of these risks, countries and international regulatory bodies, such as the Codex Alimentarius and various governmental health agencies, have established MRLs for specific drugs in food products. The control of these residues is critical not only for consumer safety but also for maintaining the integrity of food production systems globally.
This technical note summarizes AOAC Official Method 2020.04 for the screening of 152 veterinary drug residues in food-producing animals. The method has been validated through extensive testing, including single-laboratory validation (SLV) and multi-laboratory validation (MLV), showcasing its reliability and reproducibility. The detailed methods and results are published in the peer-review paper.1
Methods
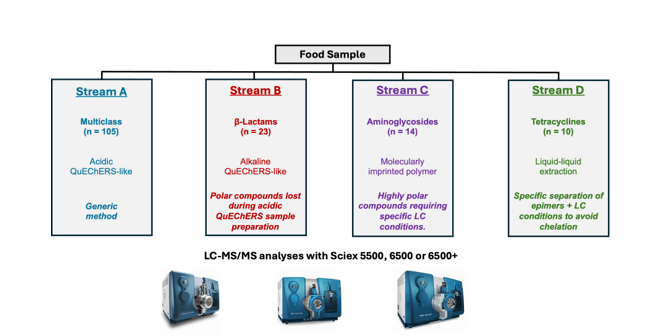
The method used a streamlined approach to analyze 152 veterinary drug residues through a series of four analytical streams, each targeting specific groups of veterinary drugs:
- Stream A – 105 veterinary drugs: Generic QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) procedure to detect antibiotics, anti-inflammatory drugs, and antiparasitic agents.
- Stream B – 23 beta-lactams antibiotics: Modified QuEChERS procedure characterized by an initial extraction step using a basic buffer solution.
- Stream C – 14 aminoglycoside antibiotics: Highly selective clean-up using molecularly imprinted polymers (MIP) combined with LC conditions tailored for polar molecules.
- Stream D – 10 tetracyclines antibiotics: Simple protocol optimized to reduce the chelation of tetracyclines and related epimers during the sample preparation and LC-MS/MS analysis.
This division into four streams optimized the method performance by tailoring the extraction and analysis procedures to each drug class. Each stream was designed to maximize the detection sensitivity while accounting for the complex matrix effects present in different food commodities.
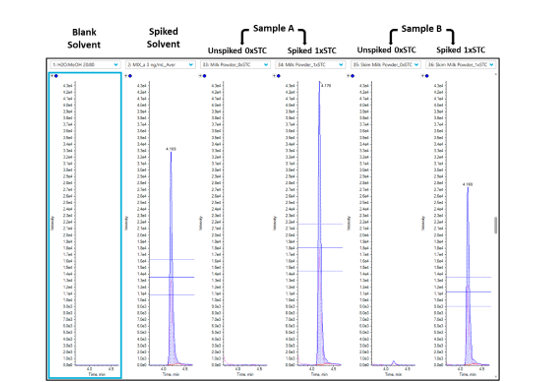
Sample preparation and processing: To compensate for losses occurring during extraction and matrix effects during MS source ionization, each individual test sample is prepared with two test portions (Figure 2):
- Test Portion 1: Unspiked test portion, to check for the presence of residues in their native state.
- Test Portion 2: Spiked with a known concentration of the target veterinary drugs at the Screening Target Concentration, or STC (≤MRLs).
LC chromatography: Full details of the LC method, along with example chromatograms, are available in Supplemental Information of the original paper. 2
Mass spectrometry: Analysis was performed using the SCIEX 5500, 6500 and 6500+ systems. The source and gas parameters, as well as the MRM tables, for the 5500 system, can be found in Supplemental Information of the original paper.1,2
Data processing: Data analysis was performed using the MultiQuant software (v3.0) with the “side-by-side” peak review option to efficiently compare unspiked versus spiked samples. Example for eprinomectin B1a in milk is shown in Figure 3.
Validation and Performance of the Method
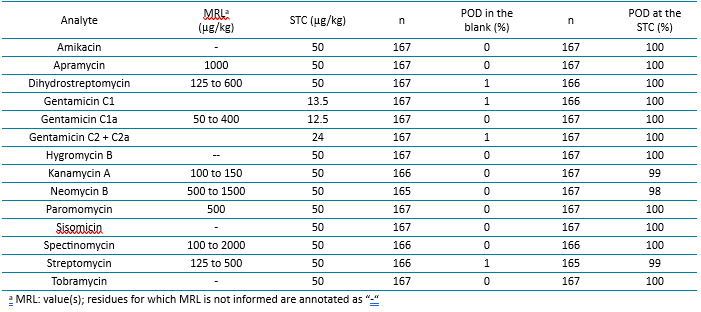
The method was evaluated through extensive validation, including single-laboratory validation (SLV) and multi-laboratory validation (MLV). The SLV demonstrated that the method could reliably detect veterinary drug residues across a wide range of food products, including dairy, meat, fish, egg-based foods, animal fat and byproducts. See Table 1 for stream C validation data. For data on all of streams, please see reference 1. The multi-laboratory validation further confirmed the method robustness by involving five independent laboratories located in Europe, Asia, and America. Each laboratory applied the method to various food matrices, and the results were consistent across all participants.
The performance of the method was evaluated based on its probability of detection (POD). The validation results show that:
- PODs at the Screening Target Concentration (STC) were greater than 94%, indicating a high likelihood of detecting residues when present.
- PODs in blank samples were less than 9%, ensuring that false positives are minimal.
- Additionally, the method was assessed through participation in 92 proficiency tests, yielding over 99% satisfactory results. This level of accuracy and reliability is critical for ensuring the safety of food products before they reach consumers (Table 1).
Method benefits
- Comprehensive detection: The method screened for 152 veterinary drug residues across a diverse range of food products, making it one of the most extensive multi-residue detection platforms available. It addressed the need for a broad scope to monitor multiple drugs within various matrices efficiently.
- High sensitivity and accuracy: LC-MS/MS technology is highly sensitive and capable of detecting residues at very low concentrations, at or below MRLs established by regulatory bodies worldwide. This ensures that even trace amounts of residues can be identified and quantified, which is essential for consumer safety.
- Robustness and reproducibility: The method's validation across multiple laboratories demonstrates its reproducibility and robustness. This is crucial for regulatory authorities and food producers who rely on consistent, repeatable results to ensure compliance with food safety standards.
- Efficient screening tool: The method functions as a qualitative screening tool, enabling food producers to rapidly assess whether veterinary drug residues are present at concerning levels. This initial screening can then be followed by more detailed quantitative testing if necessary, saving time and resources in routine monitoring validation.
- Global application and compliance support: The method was designed with the global marketplace in mind. Given that different countries have varying regulations regarding veterinary drug residues, this method supports compliance with international standards. It enables food producers to ensure that their products meet the regulatory requirements of their target markets, reducing the risk of trade barriers and food safety violations.
Impact on food safety and public health
This method, using SCIEX LC-MS/MS systems, plays a pivotal role in enhancing food safety and protecting public health by providing a reliable and efficient means of monitoring veterinary drug residues. One of the most pressing concerns related to these residues is the growing threat of antimicrobial resistance (AMR). AMR arises when bacteria become resistant to antibiotics, rendering them ineffective for treating infections in humans and animals. The overuse and misuse of antibiotics in food-producing animals can contribute to this global health crisis. By ensuring that food products are free from excessive or prohibited levels of antibiotics, this method can help combat the spread of AMR.
In addition to AMR, the method addresses other potential health risks posed by veterinary drugs, such as allergic reactions, carcinogenicity, and teratogenicity. The ability to detect a wide range of residues ensures that food products are safe for consumption, protecting consumers from the harmful effects of these substances.
Furthermore, the method provides critical support to the food industry by facilitating compliance with national and international food safety regulations. This is particularly important in a globalized food supply chain, where products cross borders and must meet the safety standards of different countries. The method helps food producers maintain high standards of quality, ensuring consumer confidence and market access
Conclusions
The method described in this technical note for screening veterinary drug residues represents a significant advancement in food safety testing. The method was developed on SCIEX instruments and has been recognized as AOAC Official Method 2020.04.1 The ability to screen for a wide range of veterinary drugs with high sensitivity and accuracy makes it an invaluable tool for the food industry and regulatory bodies alike. By providing a reliable, efficient, and comprehensive platform for detecting drug residues, the method enhances public health protection, supports regulatory compliance, and mitigates risks associated with antimicrobial resistance. Its impact on global food safety is far-reaching, helping to ensure that the food we consume is free from harmful residues and safe for everyone.
References
- Desmarchelier, A.; Bessaire, T.; Savoy, M.-C.; Tarres, A.; Mujahid, C. et al. Screening of 152 veterinary drug residues in animal source foods by LC-MS/MS, multilaboratory validation study: final action 2020.04. J. AOAC Int. 2024, 107(4), 617-631. DOI: 10.1093/jaoacint/qsae032
- Delatour, T.; Racault, L.; Bessaire, T.; Desmarchelier, A. Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit. Contam. Part A. 2018, 35(4), 633-646.
DOI: 10.1080/19440049.2018.1426890
