Abstract
In this technical note, the electron-based fragmentation type, electron activated dissociation (EAD), was used to identify the location of double
bonds and sites of branching in fatty acids using the ZenoTOF 7600 system.
The characterization of lipid isomers is a distinct challenge for lipid analysis by mass spectrometry. Collision-induced dissociation (CID), a thermal fragmentation event, typically does not provide fragments that are diagnostic of the fatty acid positions along the glycerol backbone, nor does it provide information about the position (s) of carbon-carbon double bonds within the fatty acid carbon chain. A novel fragmentation mode, EAD, generates ~15-fold more fragments than CID, and provides sufficient diagnostic fragments to routinely structurally characterize complex lipids at the lipid class, fatty acid, fatty acid position, and double bond position levels.
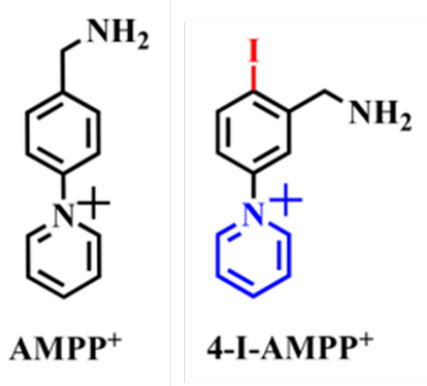
Here, the power of EAD-based fragmentation using the ZenoTOF 7600 system was used to structurally identify fatty acids (FA), focusing on the ability to identify the double bond positions of fatty acid isomers, as well as to characterize branched-chain fatty acids, using a short chromatographic timescale. The use of the novel derivatization reagent 1-(3-(aminomethyl)-4-iodophenyl)pyridin-1-ium (4-I-AMPP+), which is
shown to enhance EAD-based fragmentation (Figure 1). These results demonstrate unambiguous structural characterization of fatty acids in complex lipid extracts, including bovine milk powder, human plasma, and bacteria.
Key features of lipid mediator analysis on the ZenoTOF 7600 system
- The ZenoTOF 7600 system is capable of EAD-based fragmentation that can be used to structurally characterize fatty acid isomers
- Derivatization of fatty acids using 1-(3-(aminomethyl)-4-iodophenyl)pyridin-1-ium (4-I-AMPP+) improves sensitivity in the positive ion mode and chromatographic resolution of fatty acids by reverse phase chromatography
- EAD-based fragmentation provides diagnostic fragment ions to locate the position of double bonds and methyl branching in fatty acids
Introduction
Lipids play a crucial role in cellular biology as they are fundamental components of cell membranes, energy storage molecules, and signaling mediators. The primary structure of cell membranes—the lipid bilayer—is composed mainly of phospholipids, which create a selective barrier that regulates the movement of substances in and out of cells. Fatty acids, which are the building blocks of phospholipids, contribute to membrane fluidity and function depending on their length and degree of saturation. These fatty acids also serve as precursors for diverse lipid mediators such as prostaglandins and other enzymatically derived fatty acid oxidation products. The metabolic fate of fatty acids depends primarily on their structure, specifically the hydrocarbon chain length, number and position of double bonds, as well as the stereochemistry of carbon−carbon double bonds [1].
Gas chromatography-mass spectrometry (GC-MS) has been used to identify and quantify FAs in diverse sample types, including plasma, tissues, plant materials, oils, and seeds. GS-MS analysis requires saponification and chemical modification via derivatization before analysis; the most used derivatization technique is conversion to fatty acid methyl esters (FAME). However, the derivatization process can be time-consuming and requires authentic standards for accurate identification. Recently, EAD has been shown to be an effective tool to identify the location of fatty acid double bonds (2-4) in underivatized samples. Although this technique is effective for analyzing biological samples, a novel derivatization workflow has been proposed that enhances sensitivity and simplifies structural elucidation using EADbased fragmentation (5).
Here, fatty acids were derivatized with 1-(3-(aminomethyl)-4-iodophenyl)pyridin-1-ium (4-I-AMPP+], which incorporates both a fixed positive charge and an aryl-iodide caged-radical motif, and analyzed using the ZenoTOF 7600 system with EAD-based fragmentation. EAD mass spectra of derivatized fatty acids are dominated by [M-I],+ radical cations and product ions that result from radical-mediated dissociation of the hydrocarbon chain. Initiation of radical ion dissociation in this manner yields increases in the abundance of structurally diagnostic product ions up to 5-fold compared to underivatized fatty acids, facilitating the discrimination of isomeric unsaturated and branched-chain fatty acids. This enhancement enables the unambiguous structural assignment of fatty acids in complex lipid extracts including bovine milk powder, human plasma, and bacteria.
Materials and methods
Materials: Where possible, FAs are annotated using the recommended shorthand notation. Cis-Vaccenic acid (FA 18:1(11Z)), heptadecanoic acid (FA 17:0), and cis-nonadec-10-enoic acid (FA 19:1(10Z)) were obtained from Sigma-Aldrich (Castle Hill NSW, Australia); oleic acid (FA18:1(9Z)) and petroselinic acid (FA 18:1(6Z)) were obtained from Tokyo Chemical Industry (Tokyo, Japan); 15-methylhexadecanoic acid (FA 16:0(15Me)), 14-methylhexadecanoic acid (FA 16:0(14Me)), and cis-octadec-8-enoic acid (FA 18:1(8Z)) were obtained from Larodan (Solna, Sweden);phytomonic acid (CFA 19:0(11,12 cP)) from Sapphire Bioscience (Redfern NSW, Australia); and dihydrosterculic acid (CFA 19:0(9,10 cP)) from Adelab Scientific (Thebarton SA, Australia). Food Industry FAME mix that includes 37 fatty acids as methyl esters was purchased from RESTEK (Bellefonte, PA, USA). All solvents, reagents, and additives for LC−MS were Optima grade from Thermo Fisher Scientific (North Ryde NSW, Australia).
Sample preparation: FA standards were derivatized with AMPP+ or 4-I-AMPP+ (Figure 2) using a previously published procedure [1]. Briefly, to a solution of FA (0.088 mmol, 1.0 equiv.) in 1.0 mL of CH3CN:DMF (4:1) in a 4 mL glass vial at room temperature, was added 5 equivalents HOBt (5 mM solution in CH3CN) and 5 equivalents EDC.HCl (0.6 M in water). The resulting mixture was vortexed for 1 min and left at room temperature for 10 min before adding 5 equivalents I-AMPP+ (in 99:1 CH3CN:H2O). The resulting reaction mixture was heated at 65 °C for 1 h
before being cooled to room temperature, diluted with water (1 mL) and S43 extracted with MTBE (2 ´ 1 mL). The combined MTBE extracts were concentrated to dryness under a stream of nitrogen. The crude residue was then re-dissolved in MeOH for analysis.
Mass spectrometry: Lipid detection was performed using a SCIEX ZenoTOF 7600 system (SCIEX, Concord, CA) with electrospray ionization using Turbo Ion Source and operated in the high mass mode with positive polarity. The system parameter settings are listed in Table 2.
Data processing: LC-MS operation, data acquisition, and data processing were performed using SCIEX OS 3.1.0 (SCIEX, MA, USA).
Results
EAD-based fragmentation of AMPP+ and 4-I-AMPP+ derivatized fatty acids
The derivatizing reagents AMPP+ and 4-I-AMPP+ were initially used to improve the efficiency of fragmentation by photo dissociation (5). The ability to lock the charge of an ion at a specific location enables charge remote fragmentation. Charge remote fragmentation in mass spectrometry refers to the mechanism where bond cleavages occur at positions distant from the ionizing charge site. This process is particularly important for analyzing large and complex molecules, such as lipids. Different activation methods can induce charge remote
fragmentation. Collision-induced dissociation (CID) typically favors fragmentation near the charge site (charge-directed). Ultraviolet photodissociation (UV-PD) provides high-energy photons that can break bonds along the molecule, including remote sites, offering complementary fragmentation patterns. EAD induces charge remote fragmentation by transferring electrons that destabilize specific bonds regardless of charge location. It is hypothesized that these derivatizing reagents can improve the efficiency of EAD-based fragmentation and provide improved fatty acid structural elucidation.
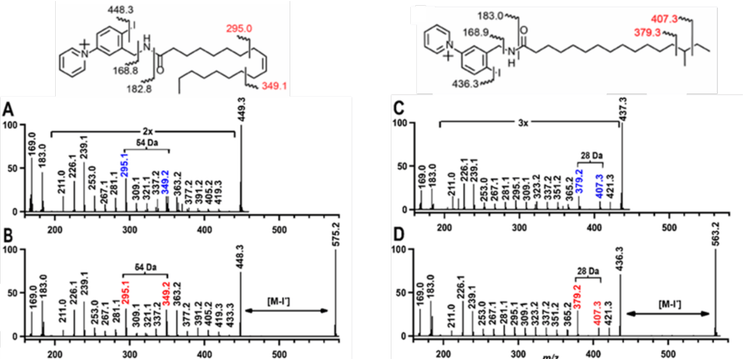
To evaluate the ability of EAD to fragment AMPP+ and 4-I-AMPP+ derivatized fatty acids, fatty acid standards were used to identify diagnostic fragments to indicate double bond position(s) or the site of alkyl chain branching. Figures 1A and B show the product ion spectra obtained from EAD-based fragmentation for a representative mono-unsaturated FA (oleic acid, FA 18:1n-9,cis), and Figures 1C and D show the fragmentation spectra of a representative branched-chain FA (iso-heptadecanoic acid, FA 16:0 (15Me)). The derivatized lipids were analyzed by ESI LC-MS/MS using EAD-based fragmentation, and the data were visualized using the Explore module of SCIEX OS.
The EAD-based fragmentation of AMPP+ FA 18:1n-9(cis) (Figure 1A) generated numerous fragment ions derived from the cleavage of carbon-carbon bonds within the acyl chain, resulting in a series of 14 Da gaps between fragments (indicating the successive loss of a methylene group) in the EAD mass spectrum from m/z 211 to 419. A distinctive and reproducible 54 Da gap was observed in the acyl chain fragment ion pattern around the site of unsaturation, which was identified as a unique marker for the localization of a double bond in the monounsaturated FA (Figure 1, left structure). Using the iodinated derivatizing agent (4-I-AMPP+) generated 4-I-AMPP-FA 18:1 (Figure 1B), This compound exhibited ion fragmentation patterns that are similar to those of AMPP-FA 18:1n-9, with the additional peak associated with the loss of iodine (M-I)• at m/z 448. As with the AMPP+ derivatives, a series of peaks differing by 14 Da was generated, ranging from m/z 211 to m/z 433, from the EAD-based fragmentation of the carbon-carbon bonds within the acyl chain. The double bond diagnostic gap of 54 Da was also observed; however, the intensities of all peaks generated by the 4-I-AMPP+ derivatives were more intense. The 4-IAMPP+ peak intensities of all structurally diagnostic peaks for FA 18:1n-9 were twice as high as those of AMPP+, suggesting the inclusion of iodine in the derivatization reagent is particularly effective for EAD-based fragmentation.
The utility of EAD-based fragmentation as a tool to identify the location of double bonds within a fatty acid led to experiments to determine whether branched-chain fatty acids can be similarly analyzed to identify the site of methylation. A branched chain fatty acid standard (FA 17:0; anteiso) was derivatized using AMPP+ or 4-I-AMPP+ derivatization and subjected to EAD-based fragmentation (Figures 1C and 1D, respectively). Both derivatives generated the carbon-chain step ladder that was observed for the unsaturated fatty acid standard.
However, rather than a gap of 54 Da, which is diagnostic of a double bond, methylation functional groups could be identified by a gap of 28
Da. In this example, there is a distinct break in the carbon chain at m/z 407 and 379, which provides the unique diagnostic peaks that indicate
the presence of the anteiso position. On the other side of the methyl branch point, radical-directed cleavage of the acyl chain backbone is consistent with the 28 Da spacing of these branching chains. In comparison to 4-I-AMPP+, the ion intensities in the AMPP+ derivative of
FA 16:0(14Me) are three times lower. These data show that derivatization of fatty acids with 4-I-AMPP+ generates diagnostic fragments for the localization of double bonds and fatty acid methylation with a high degree of sensitivity.
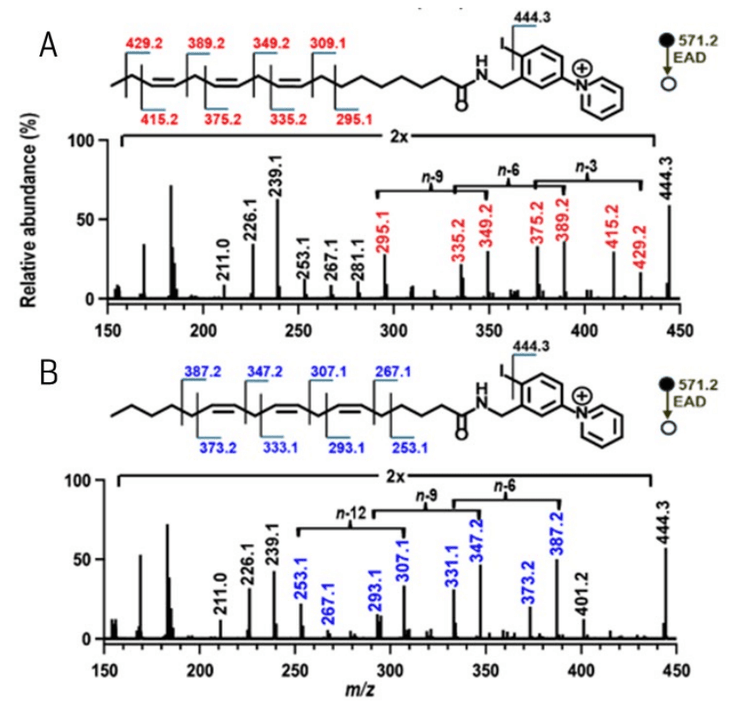
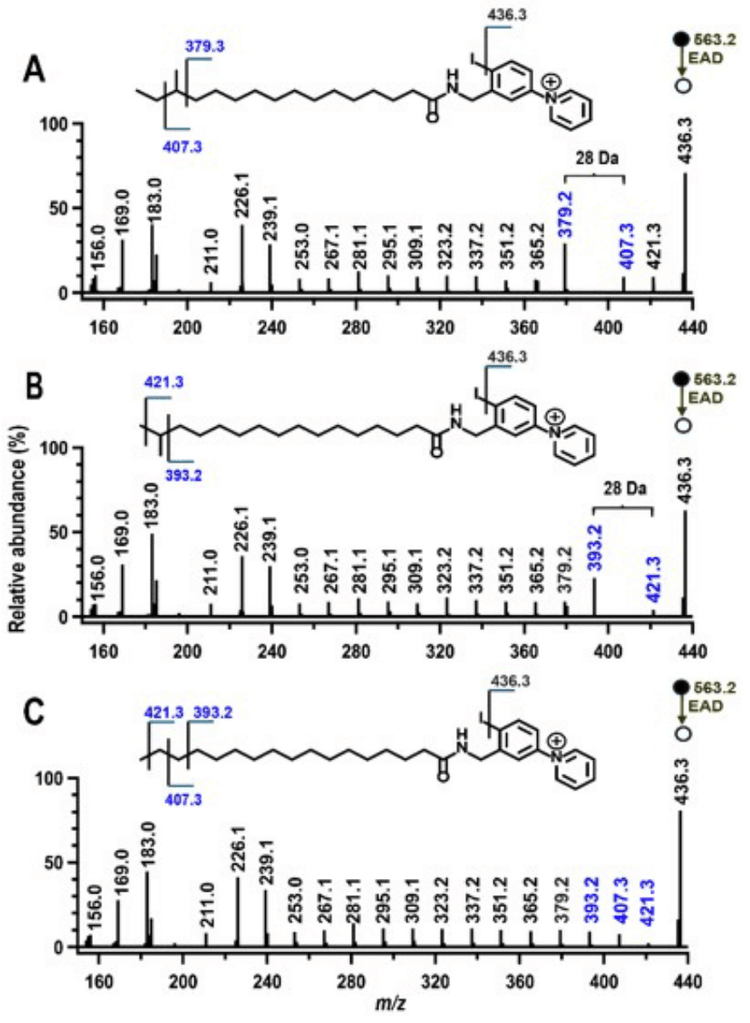
To test whether this method is equally effective for polyunsaturated fatty acids and other branched-chain fatty acid isomers, additional fatty acid standards were analyzed. For polyunsaturated fatty acids, the fatty acid isomers FA 18:3n-3,6,9 and FA 18:3n-6,9,12 were derivatized with 4-I-AMPP+ and analyzed on the ZenoTOF 7600 system using EAD-based fragmentation (Figure 3). The characteristic gap of 54 Da was observed for each double bond, indicating its position, and the two isomers are easily distinguished using this technique. Similarly, using a series of branched chain fatty acid isomers, the EADbased fragmentation spectra of the 4-I-AMPP+ derivatives of anteiso FA 16:0(14Me) (Figure 4A), iso FA16:0(15Me) (Figure 4B) and straight chain FA 17:0 (Figure 4C) display the diagnostic fragments to enable identification of the methylation site and differentiation among isomers.
In summary, the ZenoTOF 7600 system with EAD-based fragmentation is capable of dissociating fatty acids to generate diagnostic fragment
ions that correspond to sites of unsaturation and methylation within the hydrocarbon chain. Although this type of analysis is possible with
underivatized fatty acids and complex lipids (3), the use of the reagent 4-1-AMPP+ generates derivatized fatty acids that have markedly improved chromatographic resolution compared to underivatized fatty acids (not shown; see ref 5); generates 2-3 fold more intense fragment ion peaks compared to AMPP+ derivatives; and enables the structural characterization and differentiation of fatty acid isomers.
Although not shown here, the differentiation between cis and trans double bonds is also possible using EAD-based fragmentation (2).
Thus, the ZenoTOF 7600 system can provide complete characterization of fatty acids, including chain length, the number and position of double bonds, the stereochemistry of the double bond(S), and identify sites of methylation in branched chain fatty acids.
Conclusion
-
The ZenoTOF 7600 system with EAD-based fragmentation can provide for the complete structural identification of fatty acids
-
Fatty acid derivatives of 4-I-AMPP+ display improved separation with reverse phase chromatographic strategies and generate more intense structurally diagnostic fragment ions than underivatized fatty acids or those derivatized with AMPP+
-
The ZenoTOF 7600 system with EAD-based fragmentation enables:
- The localization of double bonds in mono- and polyunsaturated fatty acids
- The identification of and distinction between iso, anteiso, and straight chain fatty acid isomers
References
- de Carvalho, C. C. C. R., & Caramujo, M. J. (2018). The Various Roles of Fatty Acids. Molecules (Basel, Switzerland), 23(10), 2583. https://doi.org/10.3390/molecules23102583
- Baba, T., Campbell, J. L., Le Blanc, J. C. Y., & Baker, P. R. S. (2017). Distinguishing Cis and Trans Isomers in Intact Complex Lipids Using Electron Impact Excitation of Ions from Organics Mass Spectrometry. Analytical chemistry, 89(14), 7307–7315. https://doi.org/10.1021/acs.analchem.6b04734
- Baba, T., Campbell, J. L., Le Blanc, J. C. Y., Baker, P. R. S., & Ikeda, K. (2018). Quantitative structural multiclass lipidomics using differential mobility: electron impact excitation of ions from organics (EIEIO) mass spectrometry. Journal of lipid research, 59(5), 910–919. https://doi.org/10.1194/jlr.D083261
- Man, Z., Si, D, Baker, P.R.S., and Long, Z (2023). Identification and quantitation of unstaturated fatty acid isomers using the ZenoTOF 7600 system. SCEIX Technical Note RUO-MKT-02-15726-A.
https://sciex.seismic.com/Link/Content/DCgmW33pRfRVF8mCFgf2W4XWH8FV - Narreddula, V. R., Boase, N. R., Ailuri, R., Marshall, D. L., Poad, B. L. J., Kelso, M. J., Trevitt, A. J., Mitchell, T. W., & Blanksby, S. J. (2019). Introduction of a Fixed-Charge, Photolabile Derivative for Enhanced Structural Elucidation of Fatty Acids. Analytical chemistry, 91(15), 9901–9909.
https://doi.org/10.1021/acs.analchem.9b01566

