Accelerated pharmacokinetic profiling and metabolite monitoring Using Echo® MS System
Acoustic Ejection Mass Spectrometry
Neil Devenport1, Neil Walsh1, Simon Wood2
1SCIEX UK, 2Cyprotex Discovery UK
Abstract
Here, the Echo MS System was used for early discovery phase studies providing DMPK data in seconds as opposed to the hours required when using traditional LC-MRM based scanning methodologies. The system provided highly quantitative data with excellent %CV and accuracy. The workflow also provided clearance information with good agreement between AEMS vs traditional LC-MS as well as high-throughput metabolite monitoring enabling accurate decisions on candidate progression.

Introduction
The generation of clearance profiles and calculation of intrinsic clearance (CLint) values is an important stage in pre-clinical drug discovery.1 The pharmacokinetic (PK) assessment of potential drug candidates is typically performed by a time-course incubation with human liver microsomes (HLM) before LC-MS analysis. The in vitro CLint can be scaled to in vivo and used to predict human clearance which can be then used to inform dosing studies, as well as provide insight into enzymes responsible for metabolism of drug candidates.
As the process is performed during the early stage of drug discovery, the number of potential candidates can still be in the thousands, or even tens of thousands, and CLint values will need to be calculated for each target. This will require multiple time-points for each target leading to a large analytical demand. This creates a significant bottleneck using current LC-MS solutions.
The Echo MS System using Acoustic Ejection Mass Spectrometry (AEMS) provides a sample introduction method capable of analyzing 1 sample per second and up to 3 samples per second when multiplexed.2 With the Echo MS System, very small, reproducible droplets can be ejected from high-density well plates, and this ejection process is compatible with the wide range of solutions and complex matrices used in drug discovery. The ejected droplets are then captured by the Open Port Interface (OPI) device which uses a laminar flow of carrier solution that dilutes and transports the sample to the electrospray source, where it is ionized by conventional electrospray (Figure 2).2 This process forms a contactless sample introduction method with minimal carry-over and minimal matrix effects.
Here, the utility of this high-throughput solution was explored for use in candidate screening. The goal was to highlight the ability of the Echo MS System to alleviate the analytical bottleneck of traditional LC-MS solutions through speed and quantitative performance, and show how that correlates with current Cyprotex methodology.
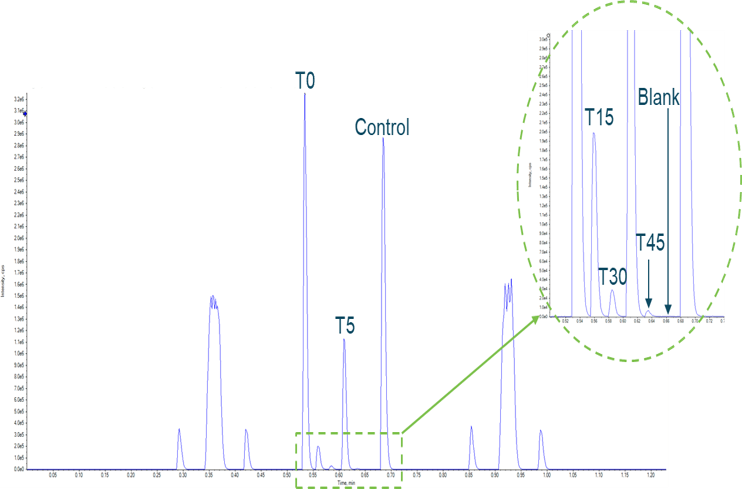
Figure 1. Microsomal incubation of buspirone. Example data using the Echo MS System were obtained from a microsomal incubation of buspirone with data acquired from 5 timepoints, a control and a blank reference, all completed in under 90 seconds. Sampling was done in a serpentine manner from the 384-well plate.
Key features of the Echo® MS System
- Rapid sample analysis using Acoustic Droplet Ejection and Open Port Interface:
- One sample per second for a single analyte, up to three samples per second in multiplex mode
- Highly reproducible sample injection
- Extremely low potential for carryover
- Broad compound coverage using electrospray ionization featuring the OptiFlow® Turbo V Ion Source for robust and efficient ionization
- Industry proven high sensitivity and quantitative robustness using the SCIEX Triple Quad™ 6500+ LC-MS/MS System
- Ease of operation using SCIEX OS Software
- Purpose built for incorporation into HTS workflows
- Sample plate tray can be accessed by most robotic arms
- Open software API for incorporation of platform into existing automated HTS environments
 Click to enlarge
Click to enlarge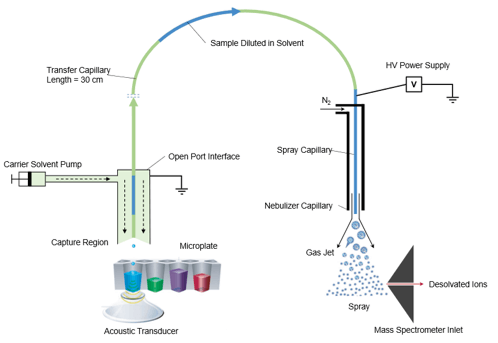 Click to enlarge
Click to enlarge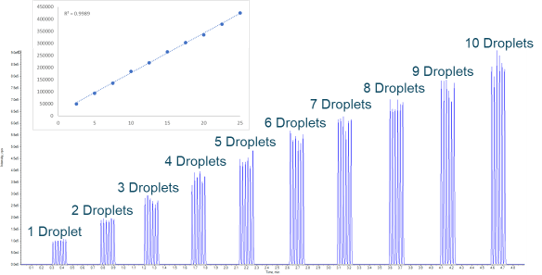 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge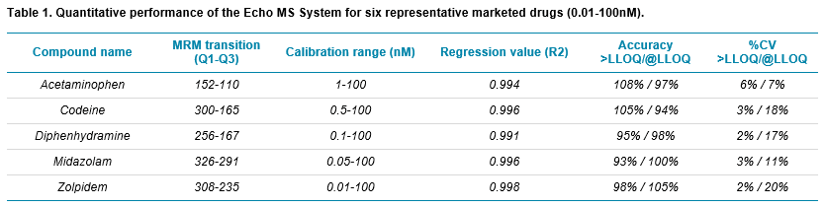 Click to enlarge
Click to enlarge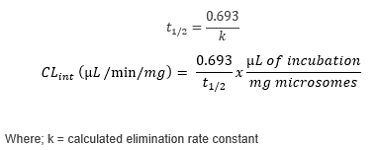 Click to enlarge
Click to enlarge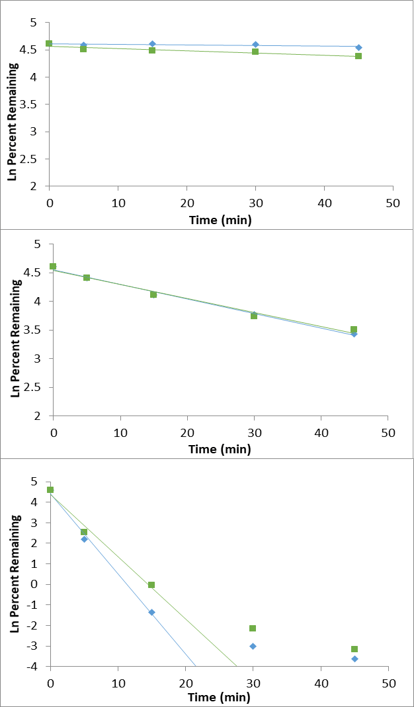 Click to enlarge
Click to enlarge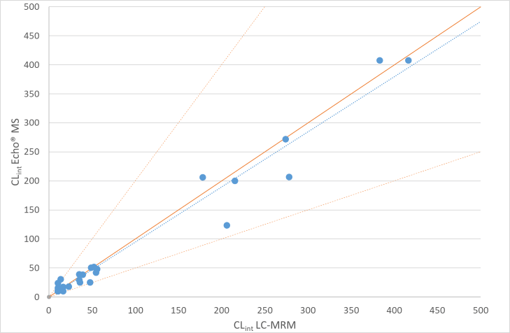 Click to enlarge
Click to enlarge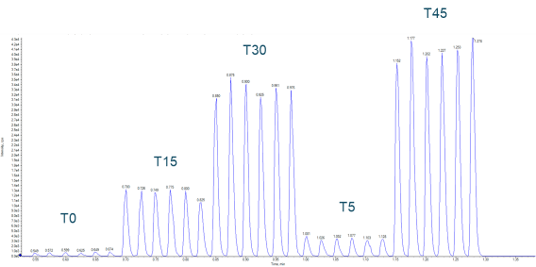 Click to enlarge
Click to enlarge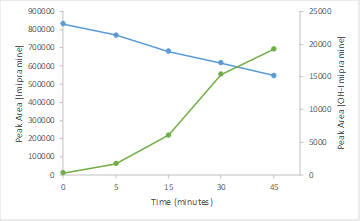 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge