Abstract
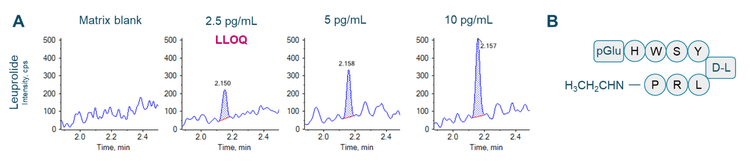
This technical note demonstrates a sensitive method to quantify leuprolide in human plasma on a SCIEX 7500 system. A lower limit of quantitation (LLOQ) of 2.5 pg/mL was determined using a 5-minute LC-MS/MS method (Figure 1).
Introduction
Leuprolide acetate is a synthetic peptide GnRH agonist that inhibits the synthesis of estrogen and androgen hormones in women and men (Figure 1B). Long-acting depot formulations have been used in suppressive treatments in men with prostate cancer, women with endometriosis and uterine fibroids and pediatric patients with central precocious puberty.1 Aside from long-acting depot formulations, leuprolide could be administered daily in microdose regimens.
Since different dosage regimens are required in hormone therapy, sensitive and selective assays for high-confidence detection and quantitative performance in biological matrices are needed to ensure the safety and efficacy of the promising therapeutic peptides.
Quantitation of peptides and proteins has been commonly performed using immunoassays, such as ELISA. However, immunoassays inherently face analytical challenges, such as poor selectivity, limited dynamic range and cross-reactivity. LC-MS/MS methods are increasingly applied for the quantitation of therapeutics as they offer the most sensitive and selective platforms for bioanalysis.
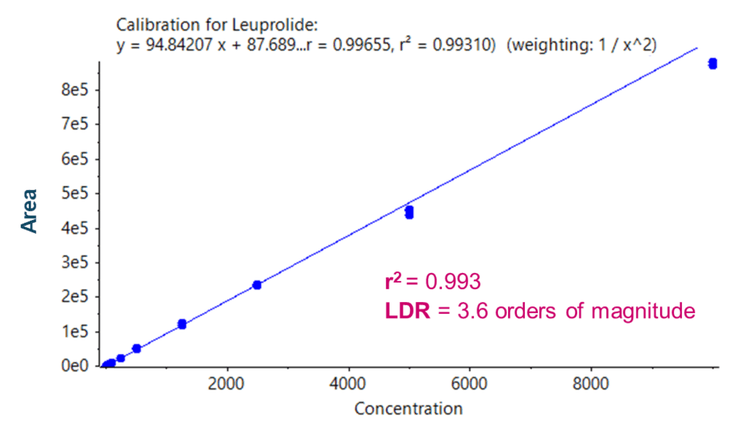
The presented method demonstrates a sensitive method to quantify leuprolide in human plasma using an efficient solidphase extraction (SPE) technique. Sensitivity at the low-pg/mL level was achieved across a linear dynamic range (LDR) spanning 3.6 orders of magnitude in human plasma using the SCIEX 7500 system. Front-end enhancements on the instrument facilitated improved overall ion generation, capture and transmission, therefore providing optimal quantitative sensitivity.
Key features of the quantitation of leuprolide using the SCIEX 7500 system
- Low-pg/mL level quantitation of a GnRH-releasing agonist: Achieve a 2.5 pg/mL LLOQ for leuprolide in human plasma on the SCIEX 7500 system
- Ideal analytical performance: Achieve accurate quantitative performance with %CV <10% at all concentration levels across a linear dynamic range (LDR) spanning 3.6 orders of magnitude
- Enhanced sensitivity unlocked: Improved front-end technology with the D Jet ion guide, OptiFlow Pro ion source and E Lens probe enhanced ion generation, capture and transmission, enabling users to reach desired quantitative sensitivity
- Streamlined data management: Data acquisition and processing are integrated into SCIEX OS software, a 21 CFR Part 11 compliance-ready platform
Methods
Sample preparation: The commercially available leuprolide (Figure 1B) was reconstituted in dimethyl sulfoxide. Individual concentrations were prepared by diluting leuprolide in a solvent containing 75:25 (v/v), water/acetonitrile with 1% formic acid and 0.05% human plasma. Individual concentrations were spiked into 100 μL of human plasma ranging from 2.5 pg/mL to 10000 pg/mL. Protein precipitation was performed with 100 μL of 5% aqueous ammonia in 90:10 (v/v), acetonitrile/water mixture. Samples were vortexed for 30 seconds and centrifuged at 12000 rcf for 10 minutes at room temperature. The supernatant was transferred to a new Eppendorf tube containing 800 μL of water and samples were briefly vortexed and centrifuged. Then, 500 μL of each sample was loaded twice on a Phenomenex Strata-X PRO microelution 96-well plate operated under positive pressure. Samples were washed with 20% acetonitrile in water. Finally, elution was performed using 50 μL of 1% trifluoroacetic acid in 75:25 (v/v), acetonitrile/water. After elution, samples were diluted with 50 μL of water after the elution to a final volume of 100 μL.
Chromatography: Sample separation was performed using an ExionLC system at a 0.6 mL/min flow rate on a Phenomenex Kinetex XB C18 (2.1 x 50 mm, 2.6 µm, 100 Å) column. Two cycles of wash in high organic with a flow rate of 1 mL/min were added for column cleaning after the elution of leuprolide (Table 1). A 5-minute gradient was run using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B (Table 1). The column temperature was maintained at 55°C. An injection volume of 10 μL was used for analysis. A mixture of 1:1:1 (v/v/v), acetonitrile/methanol/water was used as a needle wash solvent.
Quantitative performance
The circulating concentrations in human plasma for depot leuprolide injections vary between high-pg/mL to ng/mL levels.2 For lower dose regimens, achieving low-pg/mL sensitivity levels is critical for pharmacokinetic profiling of leuprolide. Here, a sensitive and robust LC-MS/MS quantitation assay with a wide LDR was achieved with a 5-min chromatographic run time.
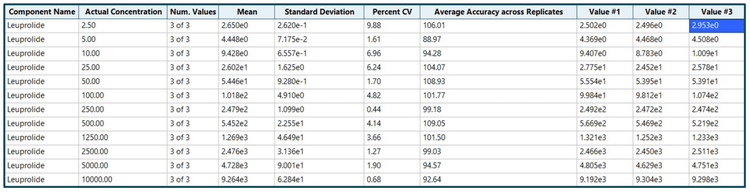
The calibration curve ranged from 2.5 pg/mL to 10000 pg/mL and was prepared as described in the sample preparation section. Individual concentrations were run in triplicate.
An LLOQ of 2.5 pg/mL was achieved for leuprolide (Figure 1). No interferences were observed in the human plasma matrix blank (Figure 1).
Linearity was achieved between 2.5 pg/mL and 10000 pg/mL with a coefficient of determination (r2 ) of 0.993 (Figure 2). An LDR spanning 3.6 orders of magnitude was achieved.
Analytical performance was evaluated for accuracy and precision. The accuracy of the calculated mean was expected to be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean for each concentration was expected to be <20% at the LLOQ and <15% at higher concentrations.
Accuracy was within ±12% of the nominal concentration and the %CV was <10% for leuprolide (Figure 3). Calculated accuracy and %CV values met the acceptance criteria at each concentration level.
Compliance-ready SCIEX OS software
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 4 illustrates the features of SCIEX OS software that are used for monitoring the audit trail, acquiring and processing data and configuring user access.
The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.

Conclusion
- An LLOQ of 2.5 pg/mL was reached for the quantitation of leuprolide in human plasma
- Linearity was achieved between 2.5 pg/mL and 10000 pg/mL, with an r2 of 0.993
- The method demonstrated accurate and highly reproducible (%CV <10%) quantitative performance at all concentrations
- Sensitivity was achieved on the SCIEX 7500 system with an improved front-end technology for better ion generation, capture and transmission
- SCIEX OS software is compliance-ready to support 21 CFR Part 11 and integrates with a triple quadrupole mass spectrometer to support data acquisition, processing and management on a single platform
References
- Kristof Chwalisz. Clinical development of the GnRH agonist leuprolide acetate depot. F S Rep. 2022 Nov 21;4(2 Suppl):33-39.
- Piero Periti, Teresita Mazzei, Enrico Mini. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41(7):485-504.


