Abstract
Exenatide is a large therapeutic peptide that enhances glucose-dependent insulin secretion by the pancreatic beta-cell, acting as a regulator of glucose metabolism and insulin secretion. Increased selectivity of MRM3 allowed for the elimination of baseline noise and chromatographic interference, resulting in superior analytical performance compared to traditional MRM.
Introduction
With increasing focus on biotherapeutics, there is greater interest in using LC-MS for the quantitative analysis of proteins and peptides in pharmaceutical research. Exenatide is a large therapeutic peptide that has been approved for the treatment of Diabetes mellitus type 1 and 2. This peptide enhances glucose-dependent insulin secretion by the pancreatic beta-cell, acting as a regulator of glucose metabolism and insulin secretion.
In recent years, the plasma concentrations of exenatide were measured by ligand-binding assays, such as immune-enzymatic assays used for pharmacokinetic studies. However, there are specificity and selectivity risks with these types of analysis since certain compounds may have similar physiochemical properties. For this reason, an MRM3 LC-MS strategy1 was explored here as a way to obtain higher selectivity in peptide using the SCIEX QTRAP® 5500 System.
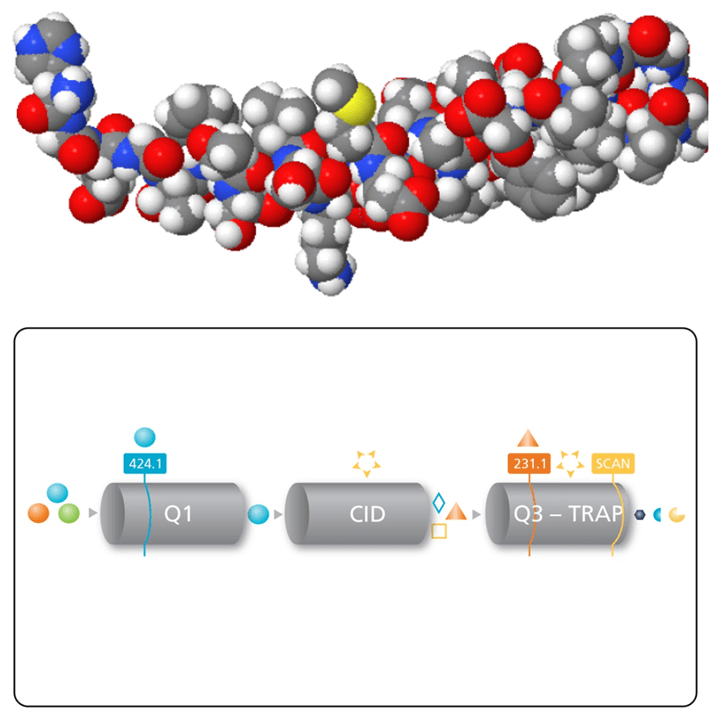
Key features of MRM3 for quantifying large therapeutic peptides
- Because of the multiple fragmentation steps used in MRM3, higher selectivity is typically achieved.
- Improvements to the QTRAP® 5500 Systems have enabled faster and more sensitivity MRM3 analysis
- Detection limits in very complex matrices can often be improved using MRM3 analysis by removing interferences at the low end of the concentration curve, improving signal/noise.
- Unlike MS3 on traditional ion traps, the unique hybrid triple quadrupole – linear ion trap design allows Q1 to be used for precursor ion selection (unit resolution), and Q2 for the first fragmentation step in a transmission mode. This allows higher speed and more flexibility in the choice of the first product ion since there is no low mass cut-off associated with the first fragmentation step in Q2, and higher collision energies can be used.
Methods
Sample preparation: Exenatide was extracted from human plasma, dried in a TurboVap under nitrogen and reconstituted. In all steps, pH values and organic phase were carefully controlled.
Liquid chromatography:
- LC: Shimadzu UFLC LC-20ACXR
- Column: Reverse phase C-18 2.0 x 30 mm, 5 µm
- Flow rate: 0.6 mL / min
- Injection volume: 5 µL
- Mobile Phase A
- 0.1% Formic acid in water
- Mobile Phase B
- 0.1% Formic acid in Methanol
- Gradient: 2 – 95% B in 5 minutes
Mass spectrometry: LC-MS analysis was done on the QTRAP® 5500 System (SCIEX) using the MRM3 acquisition strategy1. The principle of MRM3 analysis as performed on the QTRAP® 5500 System is demonstrated in Figure 1. Using the MS3 scan type, the trap was filled using Dynamic Fill Time (DFT) and the instrument was scanned at 10,000 Da/sec. The trap excitation time was 25 ms, giving a total cycle time of 0.17 sec. The transition ions used for the MRM3 analysis were 838 → 396 → 202.
Assay performance for exenatide
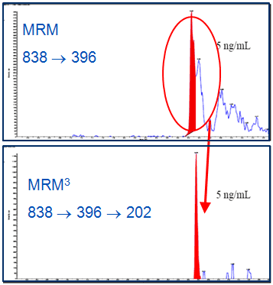
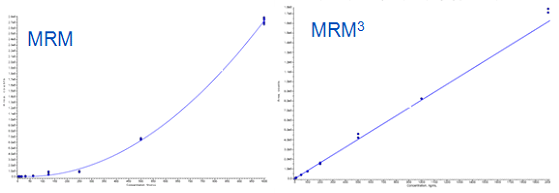
Use of MRM3 analysis resulted in significantly improved selectivity of detection for exenatide in human plasma extracts. Figure 3 shows a comparison of MRM3 vs. traditional MRM quantitation. Baseline was lower and chromatographic interference from the plasma matrix was significantly reduced in MRM3. The fast scanning speed of the QTRAP® 5500 System (10 000 Da/sec) provided a sufficient number of data points across the analyte chromatographic peak for good reproducibility of quantitation.
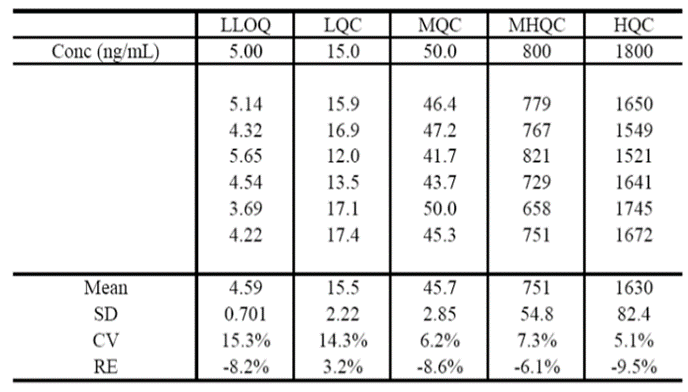
The improved detection performance resulted in excellent assay performance at the LLOQ and four QC levels as shown in Table 1. Accuracy and %CV for six replicates demonstrate that the MRM3 approach is capable of quantitative performance suitable for development-grade bioanalytical assays.
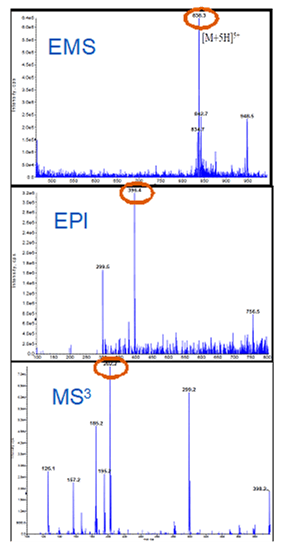
Assay development results
In Enhanced MS (EMS) mode, the multiple charged parent ion [M+5H]5+ at m/z 838.3 was selected as the first precursor (Figure 2, top). When this charge state is fragmented, the predominant product ion m/z 396.4 was chosen as the second precursor (Enhanced Product ion (EPI) scan, Figure 3, middle). The m/z 396.4 was fragmented in LIT to generate MS/MS/MS spectrum (Figure 2, bottom). The major fragment ion m/z 202.2 was selected as the second generation product ion for the MRM3 quantification.
Conclusions
- A bioanalytical assay for Exenatide in human plasma was successfully developed using MRM3 analysis.
- The increased selectivity of MRM3 allowed for the significant reduction of baseline noise and chromatographic interference, resulting in improved analytical performance compared to the MRM approach in this example.
- MRM3 demonstrated the potential for excellent linearity achieving a calibration range of 5-2000 ng/mL, compared with a range of less than 250-1000 ng/mL for traditional MRM with this analyte due to interferences.
- Accuracy and reproducibility of the MRM3 assay was compatible with the requirements for a development stage bioanalytical assay.
References
- MRM3 quantitation for highest selectivity in complex matrices. SCIEX technical note RUO-MKT-02-2739-A.