Abstract
This technical note demonstrates a sensitive method for the quantitation of AUTAC4 in human plasma using Zeno MRMHR on the ZenoTOF 7600 system. A lower limit of quantitation (LLOQ) of 0.5 ng/mL was achieved using a fast and simple sample preparation (Figure 1).
Targeted protein degradation (TPD) is an emerging therapeutic approach that offers the potential to address disease-related proteins that have traditionally been difficult to target using conventional small-molecule drugs.¹ AUTAC4 targets mitochondria by binding to the translocator protein, inducing selective mitophagy. In down syndrome fibroblasts, it showed an improvement in mitochondrial function and reduced apoptosis, demonstrating therapeutic potential for mitochondrial dysfunction.² Therefore, when assessing levels of AUTAC4 in biological matrices for pharmacokinetic and pharmacodynamics, it is crucial to employ sensitive assays to help effectively evaluate the efficacy and the safety of the drug.
Key features for analysis of AUTACs using the ZenoTOF 7600 system
- Sensitive quantitation of AUTACs: Achieve 0.5 ng/mL LLOQ for the quantitation of AUTAC4 in human plasma
- Simple sample preparation: HRMS enabled a simple protein precipitation preparation for the quantitation of AUTAC4 in human plasma with an average recovery of 70.3%
- Robust analytical performance: Achieve highly reproducible quantitative performance with %CV <11 at all concentration levels across a linear dynamic range (LDR) of 3.3 orders of magnitude
- Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplifies data acquisition and processing
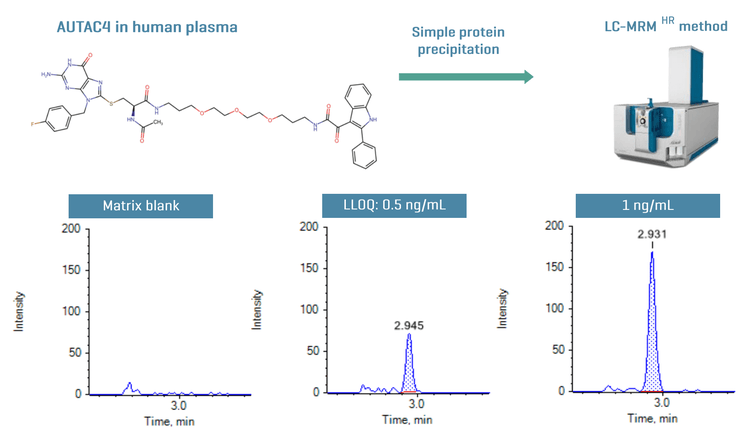
Introduction
AUTACs are synthetic molecules that trigger selective autophagic degradation by mimicking endogenous signaling. Each AUTAC contains a guanine-based degradation tag³ and a target-specific ligand connected by a PEG linker, promoting K63-linked polyubiquitination and p62-mediated autophagic clearance. AUTAC4, a mitochondria-targeting variant, uses a 2-phenylindole moiety to bind to the translocator protein on the outer mitochondrial membrane, enabling selective mitophagy. In down syndrome derived fibroblasts, AUTAC4 enhanced mitochondrial morphology, membrane potential, ATP production and reduced apoptosis, demonstrating its promise as a cargo-specific autophagy-based therapeutic for mitochondrial dysfunction.¹,²
Due to the therapeutic effect of AUTAC4 for treating degenerative diseases, highly sensitive assays are necessary to ensure precise and accurate detection and quantitation when assessing the pharmacokinetic and pharmacodynamic effects.
Methods
Standard preparation: 1 mg of AUTAC4 standard was dissolved in 1 mL of DMSO and vortexed. Further intermediate concentrations were prepared in 50:50 (v/v) methanol:water, with 0.1% formic acid as diluent.
Calibration curves (0.5–1000 ng/mL) were prepared in human plasma by serial dilution and analyzed in triplicate. Pre- and post-spiked plasma samples (in 200 μL of human plasma) were prepared at 1, 2.5, 400 and 800 ng/mL, with each concentration analyzed in triplicate.
Sample preparation: AUTAC4 was spiked into 200 μL of human plasma (0.5–1000 ng/mL). A 600 μL of methanol containing 0.1% formic acid was added to each sample and vortexed for 5 min at 2500 rpm followed by centrifugation at 15,000 rpm for 15 min. The supernatant was dried under nitrogen flow and reconstituted with 200 μL of diluent. Samples were then transferred to vials for analysis.
Chromatography: Analytical separation was conducted on an ExionLC 40 system with a Phenomenex Luna omega polar C18 column (2.1 × 50 mm, 5 μm, 100Å) at 0.3 mL/min. Mobile phase A and B were 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively. The column was kept at 45 °C. Gradient conditions are summarized in Table 1. A 3 μL sample was injected for LC-MS analysis. Flow was diverted to waste from 0–2.5 min and 3.5–6 min to prevent contamination of the mass spectrometer.
Mass spectrometry: The optimized source and gas parameters are listed in Table 2 and the Zeno MRMHR parameters are included in Table 3.
Data processing: Analysis was performed using SCIEX OS software, version 3.4.5. Peaks were integrated using the MQ4 algorithm, and a weighting of 1/x2 was used for AUTAC4 quantitation. An XIC peak width of 0.02 Da was applied.
Quantitative performance on ZenoTOF 7600 system
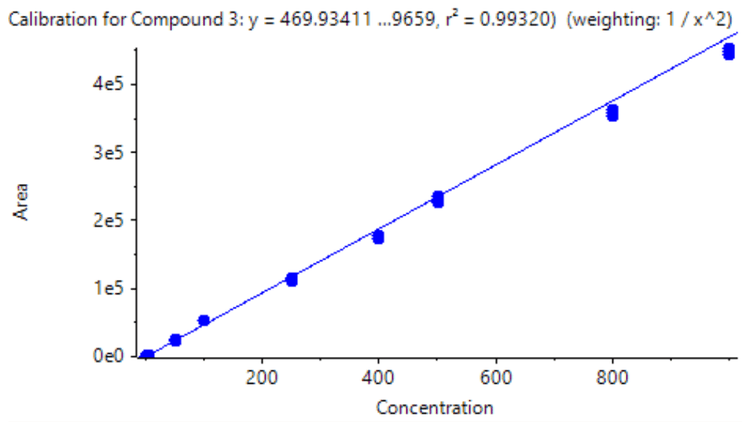
A triplicate injection was performed across concentrations ranging from 0.5 ng/mL to 1000 ng/mL (Figure 2). An LDR of 3.3 orders of magnitude was achieved. A weighing factor of 1/x2 was used and a coefficient of determination (r2) of >0.993 was reached, showing excellent linearity across a wide calibration range.
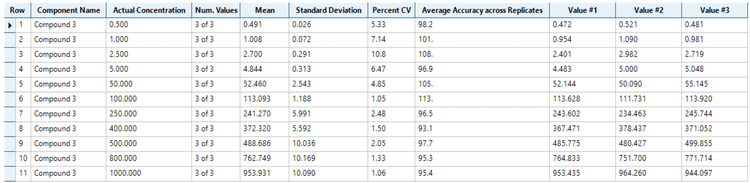
Analytical performance was evaluated based on the requirement that the accuracy of the calculated mean should be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean of the concentration should be below 20% at the LLOQ and below 15% at all higher concentrations.4
The assay accuracy was within ±13% of the nominal concentration and the %CV was <11 for the quantitation of AUTAC4 in human plasma (Figure 3). Calculated %accuracy and %CV values were within the acceptance criteria at each concentration level.
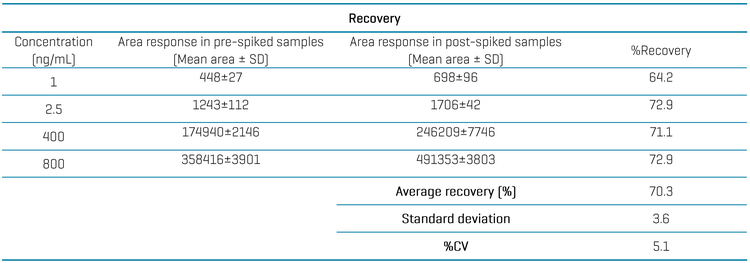
Recovery was evaluated for AUTAC4 at 4 different concentrations (1, 2.5, 400 and 800 ng/mL). A triplicate injection of the pre-spiked samples was compared to the post-spiked samples. The average recovery was 70.3% with a %CV of 5.1, achieved using a simple protein precipitation approach (Table 4).
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 7600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features. SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations.
Figure 4 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards.
The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.

Conclusion
- An LLOQ of 0.5 ng/mL was achieved for the quantitation of AUTAC4 in human plasma
- A simple sample preparation in combination with HRMS was applied to achieve a sensitive workflow for AUTAC quantitation
- Linearity was achieved at concentrations ranging from 0.5 ng/mL to 1000 ng/mL with an r2 >0.993, covering an LDR of 3.3 orders of magnitude
- Good quantitative performance was demonstrated with accurate and highly reproducible (%CV <11) results on the ZenoTOF 7600 system
- An average recovery of 70.3% was achieved with %CV <5.1 using simple protein precipitation
- Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support quantitative analysis on the ZenoTOF 7600 system
References
- Targeted protein degradation: advances in drug discovery and clinical practice, Guangcai Zhong et al. Article number: 308 (2024), 06 November 2024
- Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials, Xin Xie, Tingting Yu Xiang Li, Nan Zhang, Leonard J. Foster, Cheng Peng, Wei Huang, and Gu He 06 Sep 2023.
- AUTACs: Cargo-Specific Degraders Using Selective Autophagy, Daiki Takahashi et al.Volume 76, Issue 5, 5 Dec 2019
- Bioanalytical Method Validation, May 2018


