Abstract
Bioanalytical laboratories are constantly challenged by the need for reliable triple quadrupole mass spectrometers to ensure the delivery of proper quantitative performance. In order to effectively meet the required drug discovery and development timelines, bioanalytical workflows need robust mass spectrometers to minimize instrument downtime.
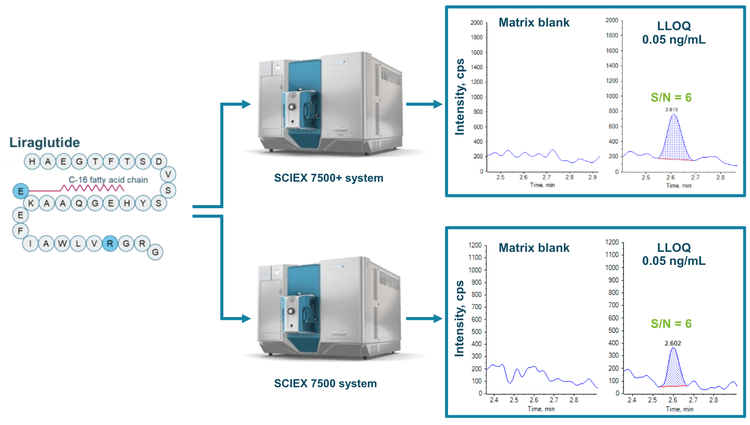
Enhanced robustness to support long-term routine bioanalysis is now feasible on the SCIEX 7500+ system with Mass Guard technology.1 Established bioanalytical workflows on the legacy SCIEX 7500 system can be seamlessly transferred to the SCIEX 7500+ system to capitalize on increased robustness while maintaining system sensitivity. In this technical note, a quantitative method for a commercially available glucagon-like peptide-1 (GLP-1) analog, liraglutide, in rat plasma was applied to demonstrate method transferability from the SCIEX 7500 system to the more robust SCIEX 7500+ system (Figure 1).
Key benefits of quantitative workflows on the SCIEX 7500+ system
- Easy method transfer with equivalent quantitative performance: A comparable LLOQ of 0.05 ng/mL and an accurate quantitative performance with a %CV <5% were achieved for the quantitation of liraglutide in rat plasma
- Wide dynamic range retained following method transfer: A wide linear dynamic range (LDR) between 0.05 ng/mL and 500 ng/mL (r2 >0.996) spanning 4 orders of magnitude was reached on the SCIEX 7500+ system
- User accessibility to the DJet+ assembly: Easily perform front-end cleaning needed to maintain system performance
- Built-in contamination check procedures in SCIEX OS software: Enables easy monitoring of instrument performance for quick troubleshooting
- Equivalent software capabilities for bioanalysis: Similar data management and compliance (21 CFR Part 11) features are available on SCIEX OS software
Introduction
LC-MS/MS assay development for bioanalysis relies on an accurate, precise and robust methodology that can withstand long-term routine runs without substantial instrument downtime. Thus, instrument reliability is imperative in meeting the arduous timelines during drug discovery and development.
The SCIEX 7500+ system features enhanced robustness using Mass Guard technology1 while maintaining unwavering quantitative comparability to the legacy SCIEX 7500 system. The presented method demonstrates quantitative method transferability from the SCIEX 7500 system to the SCIEX 7500+ system for a commercially available GLP-1 analog, liraglutide.
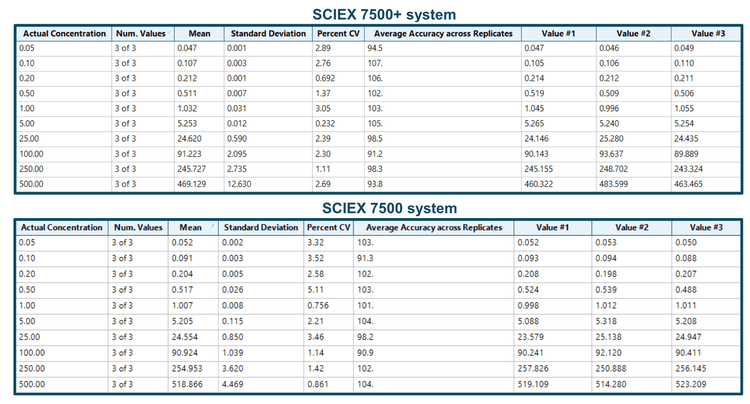
Both platforms demonstrated the same level of sensitivity at an LLOQ of 0.05 ng/mL for liraglutide in rat plasma (Figure 1). Accurate quantitative performance with a %CV <5% at all concentration levels was achieved across an LDR spanning 4 orders of magnitude on the SCIEX 7500+ system and the SCIEX 7500 system. Instrument uptime was further maintained for long-term and routine bioanalysis of GLP-1 analogs on the SCIEX 7500+ system with the user-accessible DJet+ assembly for front-end cleaning.2
Methods
The same LC conditions and MS parameters were used on the SCIEX 7500+ system and the SCIEX 7500 system. The same samples were used for analysis on both platforms.
Samples and reagents: The GLP-1 analogs liraglutide and semaglutide (IS) were purchased from Cayman Chemical Company. Liraglutide and semaglutide were reconstituted in 3% and 6% formic acid in methanol, respectively.
Sample preparation: Liraglutide was spiked into 100 μL rat plasma at concentrations ranging from 0.05 to 500 ng/mL. Semaglutide was used as an internal standard (IS) and spiked at 4 ng. Protein precipitation was performed with 300 μL of methanol. Samples were vortexed for 30 seconds and centrifuged at 12000 rcf for 12 minutes at room temperature. A 300 μL aliquot of 10% aqueous ammonia was added to the supernatant and gently mixed. A 600 μL aliquot of the sample volume was transferred to an anion exchange Oasis MAX μElution plate. Samples were washed with 5% ammonia in 1:1 (v/v) methanol/water.A second wash was performed using 1:2:2 (v/v/v) wateracetonitrile. Finally, elution was performed using 6% formic acid in 1:2:2 (v/v/v) wateracetonitrile mixture. The final elution volume was 100 μL. An injection volume of 2 µL was used for analysis.
Chromatography: Analytes were separated using a Phenomenex Kinetex C18 (2.1 x 50 mm, 2.6 µm, 100 Å) column at a temperature of 50°C. The ExionLC AE system was operated at a 0.6 mL/min flow rate (Table 1). Analysis was performed on a nominal mass spectrometer in positive mode. Collision energy, source and MS parameters were optimized for MRM-based quantitation.
Quantitative performance on the SCIEX 7500+ system
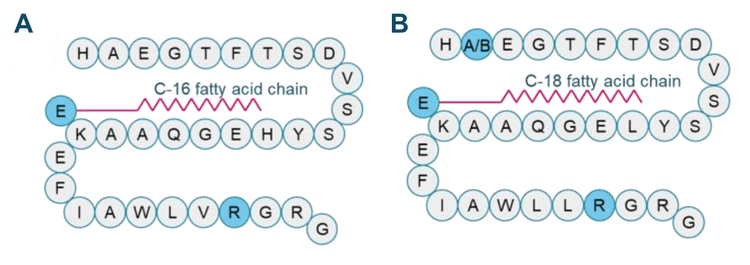
Liraglutide (Figure 2A) was quantified in rat plasma with the presence of semaglutide (Figure 2B) as an IS. Method transfer to the SCIEX 7500+ system was performed with the same LC conditions and MS parameters as on the legacy SCIEX 7500 system.
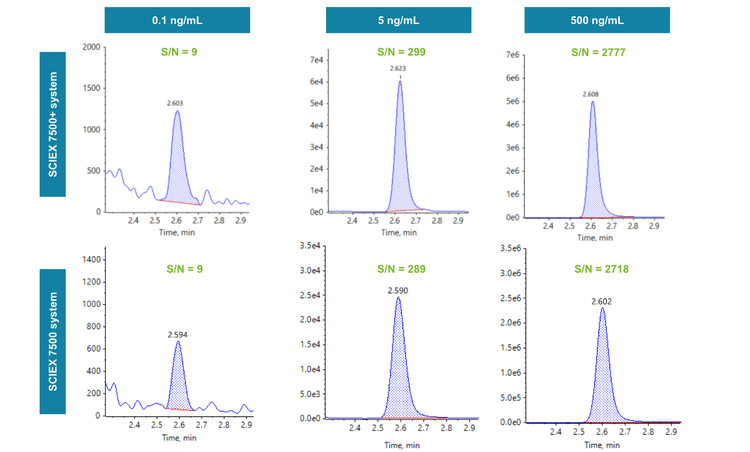
An LLOQ of 0.05 ng/mL was achieved for liraglutide on the SCIEX 7500+ system and the SCIEX 7500 system (Figure 1). No interferences were observed in the rat plasma matrix blank. Representative XICs of liraglutide in rat plasma at 0.1 ng/mL, 5 ng/mL and 500 ng/mL were also assessed between the MS platforms (Figure 3). S/N (peak-to-peak calculation) was applied to evaluate the sensitivity comparability of the SCIEX 7500+ system to the SCIEX 7500 system. At 0.1 ng/mL, a S/N of 9 was achieved on both MS systems. For liraglutide at 5 ng/mL, a S/N of 299 was reached on the SCIEX 7500+ system, and a S/N of 289 was reached on the SCIEX 7500 system. At a concentration of 500 ng/mL, a S/N of 2777 was acquired on the SCIEX 7500+ system, and a S/N of 2718 was acquired on the SCIEX 7500 system (Figure 3). Therefore, equivalent quantitative sensitivity was demonstrated on the SCIEX 7500+ system compared to the previous generation SCIEX 7500 system.
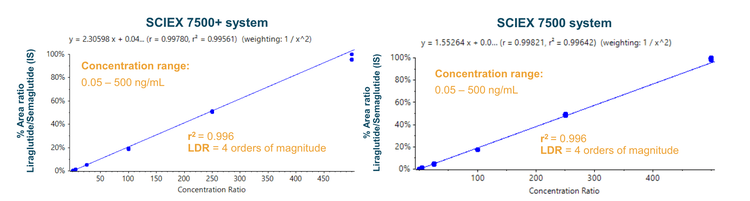
An r2 >0.996 was achieved across the 0.05 to 500 ng/mL concentration range with an LDR spanning 4 orders of magnitude on both MS platforms (Figure 4). As a result, a comparable wide quantitative range can be analyzed using the SCIEX 7500+ system.
Analytical performance was evaluated for accuracy and precision. The accuracy of the calculated mean was expected to be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations
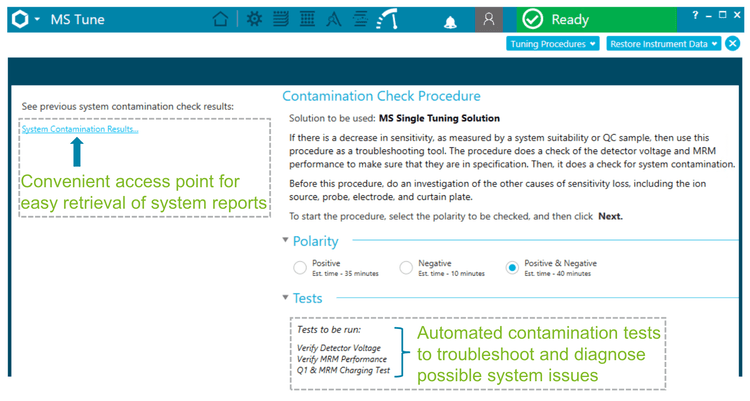
Enhanced software tools for monitoring system performance
The SCIEX OS software provides a built-in automated workflow that enables the user to monitor the detector performance and system charging events with minimal manual intervention (Figure 6). The contamination check procedure enables system tests to be run in both the positive and negative polarities using the MS single tuning solution.
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the SCIEX 7500+ system, ensuring high fidelity when performing method transfers while retaining critical compliance features.
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 7 illustrates the features of SCIEX OS software that are used to monitor the audit trail, acquire and process data, and configure user access.
The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.

Conclusion
- Equivalent LLOQ (0.05 ng/mL) was achieved for quantitation of liraglutide on the SCIEX 7500+ system compared to the SCIEX 7500 system, highlighting retention of assay sensitivity over a method transfer
- An identical quantitative range with a linear range between 0.05 ng/mL and 500 ng/mL (r2 >0.996) and an LDR spanning 4 orders of magnitude was achieved on the SCIEX 7500+ system compared to the SCIEX 7500 system
- Comparable quantitative performance was demonstrated with accurate and highly reproducible (%CV <5%) results on the SCIEX 7500+ system compared to the SCIEX 7500 system
- The combination of SCIEX OS software enhancements for system performance tracking and the extractable DJet+ assembly offers increased flexibility for user-initiated management of system maintenance and uptime
- Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support non-regulated and regulated bioanalysis on the SCIEX 7500+ system
References
- Redefine bioanalysis with enhanced robustness on the SCIEX 7500+ system. SCIEX technical note, MKT-31350-A.
- Build resilience with the SCIEX 7500+ system. SCIEX brochure, MKT-31468-A.


