Abstract
This technical note provides insight into a versatile and valuable new tool for plasmid purity analysis. The DNA 20 kb Plasmid and Linear kit is compatible with pre-built capillary cartridges, enhancing efficiency and robustness. It enables high-quality separation with purity percentages (purity%) and consistent plasmid isoform profiles similar to those obtained with the dsDNA 1000 kit on the PA 800 Plus system, ensuring reliable assay transfer between kits. It also supports method transfer between the single-capillary PA 800 Plus system and the multicapillary BioPhase 8800 system, providing consistent peak profiles, migration times (MTs) and purity% across both platforms while accommodating various throughput requirements. The kit offers easy-to-use, high-resolution plasmid DNA topology analysis across a wide size range (2.7–18.9 kb), efficiently separating supercoiled (SC) isoforms from other topological isoforms and impurities for comprehensive and efficient characterization of plasmids.
Key plasmid analysis features of the DNA 20 kb Plasmid and Linear kit
- Results comparable to those obtained with the dsDNA 1000 kit: achieves purity% and consistent plasmid isoform distributions similar to those obtained with the dsDNA 1000 kit on the PA 800 Plus system, validating its effectiveness and ensuring seamless assay transfer
- Seamless method transfer between single-capillary (PA 800 Plus system) and multi-capillary (BioPhase 8800 system) systems: demonstrates consistent peak profiles, MTs and purity% on both platforms
- Ease of use and versatility for high-resolution plasmid DNA topology analysis: enables high-resolution analysis of plasmids across a wide size range (2.7–18.9 kb), effectively separating topological isoforms with ready-to-use gel and pre-built capillary cartridges (Figure 1)
Introduction
Plasmid DNA is a fundamental starting material in cell and gene therapy manufacturing processes, including messenger RNA (mRNA) production and the generation of viral vectors or gene-of-interest (GOI) plasmids.1 Additionally, plasmid DNA is used as an active pharmaceutical ingredient (API) for protein production. Analyzing plasmid topology and purity is essential for establishing a robust plasmid DNA quality control strategy.2
Plasmid purity analysis involves assessing the presence of various topological forms, such as SC, open circular (OC), linear and others.3 It is critical to monitor the percentage of total SC plasmid DNA relative to lower amounts of other forms. High-resolution techniques are necessary to accurately distinguish these forms and ensure that plasmid preparations meet the required quality standards. Advanced analytical methods, such as capillary electrophoresis (CE), offer automated rapid and precise separation of plasmid isoforms.
The dsDNA 1000 kit is a commonly used method for plasmid topology analysis on CE systems from SCIEX. It involves reconstituting lyophilized gel powder to prepare the gel buffer, manually diluting the gel for optimized separation of plasmid samples and assembling the cartridge with the coated DNA capillary. These steps can be time-consuming, delay analysis and time to result and are prone to user errors.
This technical note introduces the DNA 20 kb Plasmid and Linear kit, a new plasmid analysis kit that features ready-to-use reagents and compatibility with pre-built bare-fused silica (BFS) capillary cartridges, providing rapid and high-resolution analysis of plasmids across a wide size range (2.7–18.9 kb). In the analysis of 5 plasmids 2.7–18.9 kb in size, the new kit achieved purity results similar to those obtained with the dsDNA 1000 kit, validating the effectiveness of the new method and streamlining the transition to the new DNA 20 kb Plasmid and Linear kit.
The performance of the new kit was evaluated on both the single-capillary PA 800 Plus system and the multi-capillary BioPhase 8800 system and compared. The kit demonstrated consistent peak profiles, MTs and purity% across both platforms, enabling smooth method transfer and the ability to meet varying throughput requirements. Additionally, a titration experiment was conducted to determine the optimal plasmid concentration for topological isoform analysis using the new kit, ensuring accurate and reliable results. The 8-capillary BioPhase 8800 system facilitates rapid titration curve optimization by simultaneously analyzing samples at different dilution points in a single run. The reliability of the new DNA 20 kb Plasmid and Linear kit makes it suitable for use at various stages of biotechnological and biopharmaceutical processes to help ensure the integrity and quality of plasmid DNA
Methods
Materials: The DNA 20 kb Plasmid and Linear kit (P/N: 5311708) was from SCIEX (Framingham, MA) and contained DNA 20 kb Plasmid and Linear gel, DNA 20 kb Plasmid and Linear sample buffer, DNA 20 kb Plasmid test mix, SYBR™ Gold Nucleic Acid gel stain*, DNA 20 kb Plasmid and Linear conditioning solution, 00.1 N HCl and CE Grade water. The BioPhase BFS capillary cartridge - 8 x 30 cm (P/N: 5080121) and BioPhase sample and reagent plates (4,4,8) (P/N: 5080311) were from SCIEX. Rainin LTS filter tips were from Mettler Toledo (Oakland, CA). Nuclease-free water (NFW) (P/N: AM9932) was obtained from Thermo Fisher Scientific (Waltham, MA). The dsDNA 1000 kit (P/N: 477410) and the pre-assembled capillary cartridge (P/N: A55625) were from SCIEX. A 10x concentrated Tris Borate EDTA stock solution (10x TBE) (P/N: T4415) was from Sigma-Aldrich (St. Louis, MO).
The 2.7 kb pUC19 Vector plasmid DNA (P/N: N3041S) at 1,000 µg/mL was from New England Biolabs (NEB) (Boston, MA). The 4.4 kb pBR322 plasmid DNA (P/N: SD0041) at 0.5 µg/µL and the 7.9 kb plasmid pCMV·SPORT-βgal (P/N: 10586014) was from Thermo Fisher Scientific (Carlsbad, CA). The 5.8 kb gWiz-GFP plasmid DNA (P/N: 5006) and the 18.9 kb pALD-X80 AAV Helper plasmid (P/N: 5017-10) were from Aldevron (South Fargo, ND).
Sample preparation for analysis using the DNA 20 kb Plasmid and Linear kit: The plasmid DNA sample was thawed on ice for about 20 minutes and diluted in the DNA 20 kb Plasmid and Linear sample buffer to a final concentration of 1 ng/μL. The diluted plasmid sample was then transferred at 100 μL per well to the sample plate for analysis on the BioPhase 8800 system or 100 μL per sample vial for analysis on the PA 800 Plus system
Sample preparation for analysis using the dsDNA 1000 kit: Plasmid samples were diluted with 1x TBE buffer to 8 ng/μL for analysis, and then 100 μL of samples were transferred to a sample microvial for analysis on the PA 800 Plus system.
Gel buffer preparation using the dsDNA 1000 kit: To rehydrate the gel buffer, 20 mL of 0.2 μm filtered deionized water was added to the gel buffer vial. The mixture was gently stirred with a small stirring bar for up to 24 hours or until the dried gel was completely dissolved. The hydrated gel is stable for 1 month if stored at 4°C. Then, the gel solution was diluted 1:10 with 1x TBE (90mM tris, 90mM borate and 2mM EDTA at pH 8.3). The diluted gel was filtered through a 0.45 μm filter. The 1x TBE was prepared by diluting the 10x TBE buffer stock solution 10 times with deionized water. The resulting buffer solution was filtered with a 0.2 μm filter. SYBR™ Gold Nucleic Acid gel stain was added to the gel at 100 μL in 10 mL gel.
Cartridge assembly using the dsDNA 1000 kit on the PA 800 Plus system: The DNA capillary was installed according to the instructions (P/N: 726412) in the dsDNA 1000 kit. The total capillary length was 40.2 cm, with 30 cm as the length to the detection window
Instrument and software: The BioPhase 8800 system—equipped with laser-induced fluorescence (LIF) detection utilizing an excitation wavelength of 488 nm and an emission wavelength of 520 nm—was from SCIEX. Data acquisition and analysis were performed using BioPhase 8800 software 1.2 from SCIEX. A PA 800 Plus system—equipped with LIF detection and solid-state laser with excitation wavelength at 488 nm and a 520 nm bandpass emission filter—was from SCIEX. Data acquisition and analysis were performed using 32 Karat software 10.2.
Instrument analysis methods: Samples prepared for analysis using the DNA 20 kb Plasmid and Linear kit were analyzed on the BioPhase 8800 system and the PA 800 Plus system to assess the comparability between these 2 platforms. The methods used on the BioPhase 8800 system were provided in the DNA 20 kb Plasmid and Linear kit application guide,4 while the methods for the PA 800 Plus system are shown in Figure 2. The BioPhase BFS capillary cartridge - 8 x 30 cm was used for analysis on the BioPhase 8800 system, while the pre-assembled capillary cartridge was used for analysis on the PA 800 Plus system.
Samples prepared for analysis using the dsDNA 1000 kit were analyzed on the PA 800 Plus system. The methods used were described in a previously published technical note.5 A coated DNA capillary at 40 cm total length (30 cm effective length) was used for analysis using the dsDNA 1000 kit on the PA 800 Plus system.
Results and discussion
Comparison of plasmid purity analysis using the dsDNA 1000 kit and the DNA 20 kb Plasmid and Linear kit: The dsDNA 1000 kit has been used for plasmid purity analysis on the PA 800 Plus system for over 10 years and is a gold standard analytical method. However, with this method, the gel must be reconstituted and the gel dilution factor must be optimized based on the size of the plasmid to analyze. Additionally, the assembly of the cartridge with the coated DNA capillary must be done manually, which can be time-consuming and prone to user errors. The DNA 20 kb Plasmid and Linear kit, on the other hand, provides a ready-to-use, pre-optimized gel that works for plasmids across a wide size range. This kit also includes pre-built capillary cartridges, which significantly enhances efficiency, reduces variation and minimizes manual preparation. The streamlined process of the new kit results in a notable reduction in method time, allowing for faster and more reliable plasmid analysis.
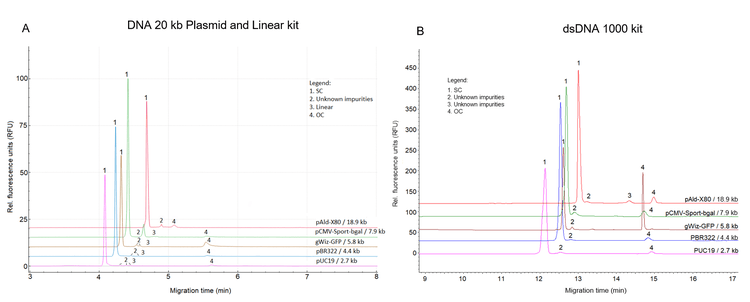
This study compared the purity analysis of 5 different plasmids in a size range of 2.7–18.9 kb using the DNA 20 kb Plasmid and Linear kit and the dsDNA 1000 kit on the same PA 800 Plus system. Figure 3A shows results obtained using the DNA 20 kb Plasmid and Linear kit. Figure 3B shows the separation of topological isoforms of the identical 5 plasmids using the dsDNA 1000 kit on the same system. The signal observed in Figure 3B differs from that in Figure 3A, which can be attributed to differences in sample concentration and dye concentration in the gel between the 2 kit methods. Additionally, the DNA 20 kb Plasmid and Linear kit employs a different capillary with a distinct internal diameter at the detection window compared to the dsDNA 1000 kit. These variations in experimental conditions contribute to the differences in signal intensity shown in Figure 3A and Figure 3B.
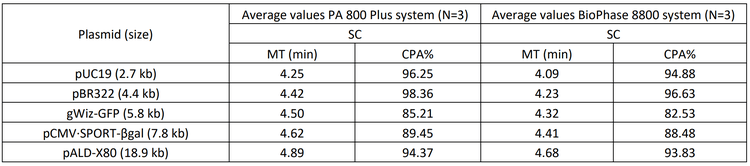
Despite these differences, the overall profiles and purity assessments remain comparable, demonstrating the robustness and reliability of both methods. Table 1 summarizes the average plasmid purity in the percentage of SC (SC%) from 3 replicate analyses of each of the 5 plasmids using the dsDNA 1000 kit and the DNA 20 kb Plasmid and Linear kit on the same PA 800 Plus system. Results in this table further demonstrate that comparable purity levels were obtained with both kits, indicating that each method effectively identifies and quantifies impurities in the plasmid samples. This ensures reliable and consistent results across different plasmid sizes.
The new DNA 20 kb Plasmid and Linear kit offers several advantages over the dsDNA 1000 kit. It features a ready-to-use, non-lyophilized gel and is compatible with pre-built BFS capillary cartridges, significantly simplifying the assay process and reducing setup time. In addition, the DNA 20 kb Plasmid and Linear kit achieves a faster separation time of approximately 10 minutes per sample compared to 25 minutes per sample with the dsDNA 1000 kit. This reduction in separation time enhances efficiency, making the DNA 20 kb Plasmid and Linear kit an ideal solution for high-quality plasmid purity analysis of larger sample batches. These benefits make it a superior choice for laboratories seeking streamlined plasmid purity assessments without compromising data accuracy or reliability.
Overall, this work demonstrates the similarity and comparability of the profiles and purity assessments of plasmids using either the dsDNA 1000 kit or the DNA 20 kb Plasmid and Linear kit on the PA 800 Plus system. The remarkable consistency in plasmid isoform distributions ensures assay portability and ease of transfer. The DNA 20 kb Plasmid and Linear kit reduces lab work, making it an efficient option for plasmid analysis.

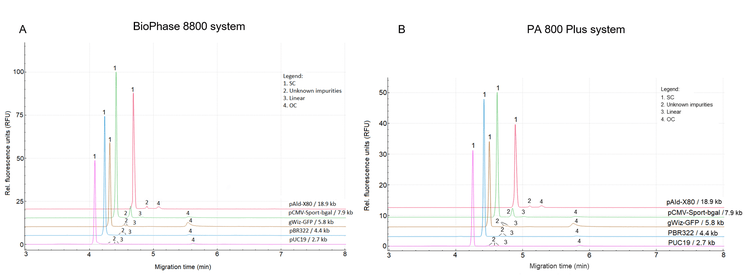
Method transfer between the single-capillary PA 800 Plus system and the multi-capillary BioPhase 8800 system using the DNA 20 kb Plasmid and Linear kit: Throughput needs often differ at different stages of drug development and manufacturing. Method transferability is crucial for laboratories needing to scale operations without compromising results. To assess the transferability of plasmid purity analysis using the DNA 20 kb Plasmid and Linear kit between the 8-capillary BioPhase 8800 system and the single-capillary PA 800 Plus system, 5 plasmids were analyzed on both systems. The samples were analyzed in parallel on the BioPhase 8800 system (Figure 1A), while the same plasmids were analyzed sequentially in 5 different runs on the PA 800 Plus system (Figure 1B). Results in both panels show the baseline resolution of the main product, the SC isoform, from other isoforms and impurities for all 5 plasmids with sizes of 2.7–18.9 kb. In addition, the peak profiles for each plasmid were consistent between the 2 systems.
Table 2 summarizes the average MT and the plasmid purity in the corrected peak area percentages (CPA%) of the SC isoform from 3 replicate analyses. With both systems, the same trend of longer MTs for larger plasmid sizes was observed with similar time differences between different plasmids. The CPA% values of the SC isoform were highly similar between the 2 systems, demonstrating consistent purity assessments of the plasmid samples. This consistency is vital for reliable and reproducible results, allowing laboratories to switch between systems without compromising assay accuracy.
In summary, the DNA 20 kb Plasmid and Linear kit method can be seamlessly transferred between the PA 800 Plus system and the BioPhase 8800 system. This ensures consistent and reliable plasmid purity assessments between labs with varying throughput requirements. Consistent peak profiles, MTs and purity% across both systems demonstrate the robustness of the method and its ability to serve as a versatile and scalable solution for plasmid purity analysis in laboratories.
Determination of the optimal plasmid concentration for plasmid purity analysis using the DNA 20 kb Plasmid and Linear kit: Optimizing plasmid concentration is critical for accurate topological isoform analysis using the DNA 20 kb Plasmid and Linear kit. A titration experiment was performed using the multicapillary BioPhase 8800 system to efficiently determine the optimal concentration. The system facilitates rapid screening, making it an ideal tool for initial optimization before transitioning to the PA 800 Plus system.
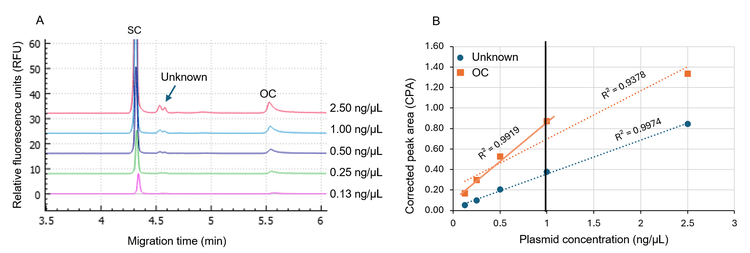
As an example, the gWiz-GFP (5.8 kb) plasmid was serially diluted from 2.50 ng/µL to 0.13 ng/µL with the DNA 20 kb Plasmid and Linear sample buffer, and the samples were analyzed simultaneously on the BioPhase 8800 system. Results were analyzed using the BioPhase software with the minimum S/N set at 10. As illustrated in Figure 4A, overlaid traces of the gWiz-GFP plasmid at different dilutions were obtained. The CPAs of unknown impurities and OC isoform were plotted against plasmid concentration (Figure 4B). The plot shows that the CPA of the unknown impurity has a good linear relationship with the plasmid concentration in the range of 0.13–2.50 ng/µL with an R2 value of 0.9974 (blue dotted line). In the same plasmid concentration range, the OC signal was linear between 0.13 ng/μL and 1 ng/μL but became saturated at 2.5 ng/μL. Therefore, the plasmid concentration range of 0.13–1.00 ng/µL was determined as a good concentration range for this plasmid sample, with the 1.00 ng/μL concentration as the optimal concentration due to its strong signal. This rapid titration experiment, conducted within 10 minutes of separation time, efficiently determines the optimal plasmid concentration for topological isoform analysis, ensuring accurate and reliable results.
Conclusion
-
Comparative analysis results: the DNA 20 kb Plasmid and Linear kit obtains purity% results similar to those obtained with the dsDNA 1000 kit, validating the effectiveness and reliability of the new method
-
Consistent results across systems: 5 different sizes of plasmids were analyzed on both single-capillary and multicapillary systems, resulting in:
- Similar peak profiles
- o Consistent migration times
- o Comparable purity%
-
Seamless method transfer: facilitates efficient method transfer across different stages of the manufacturing process, accommodating varying throughput requirements
-
Versatile capabilities: the ready-to-use DNA 20 kb Plasmid and Linear kit enables rapid and high-resolution analysis of plasmids ranging from 2.7 kb to 18.9 kb
-
Comprehensive solution: the DNA 20 kb Plasmid and Linear kit offers a high-resolution, reliable, adaptable solution for plasmid analysis that is suitable for high-quality plasmid characterization and quality control
References
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 2011 May;12(5):316–328.
- Samulski RJ, Muzyczka N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 2014 Nov;1(1):427–451.
- Goyon A et al. Separation of Plasmid DNA Topological Forms, Messenger RNA, and Lipid Nanoparticle Aggregates Using an Ultrawide Pore Size Exclusion Chromatography Column. Anal Chem. 2023 Oct 10;95(40):15017–15024.
- DNA 20 kb Plasmid and Linear kit for the BioPhase 8800 system. SCIEX application guide, RUO-IDV-05- 15737-A.
- Optimization of a Robust and Sensitive Capillary Electrophoresis-Laser Induced Fluorescence Method for Monitoring Plasmid Stability and Purity. SCIEX technical note, RUO-MKT-02-9310-A.