Advance the analysis of plasmid DNA and linear DNA to support the development of messenger RNA (mRNA), viral vectors and proteins.
Plasmid and DNA analysis

Overview
Plasmid and DNA analysis challenges can be left behind.
Whether you need high-purity supercoiled plasmid DNA (pDNA) for protein and viral vector manufacturing or linear double-stranded DNA (dsDNA) for in vitro transcription (IVT), you and your team can rely on high-quality topology and stability assessment of pDNA with SCIEX solutions.
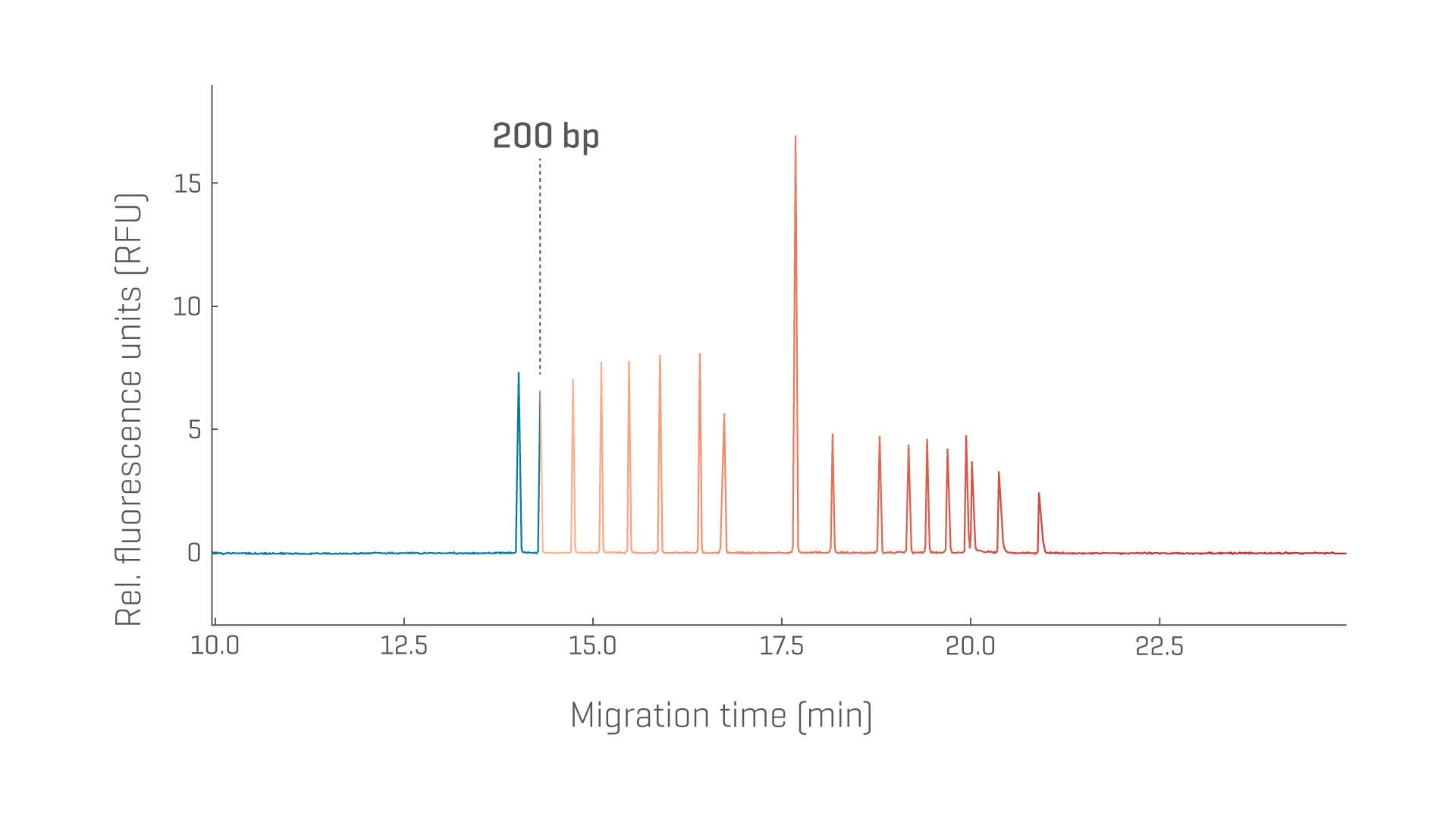
Restriction enzyme digestion of pDNA paired with ultra-high separation power enables the determination of genes of interest and long repetitive sequences.
Residual DNA assessment from cell-culture-derived products can be achieved with the same solution.
pDNA isoforms
Workflow
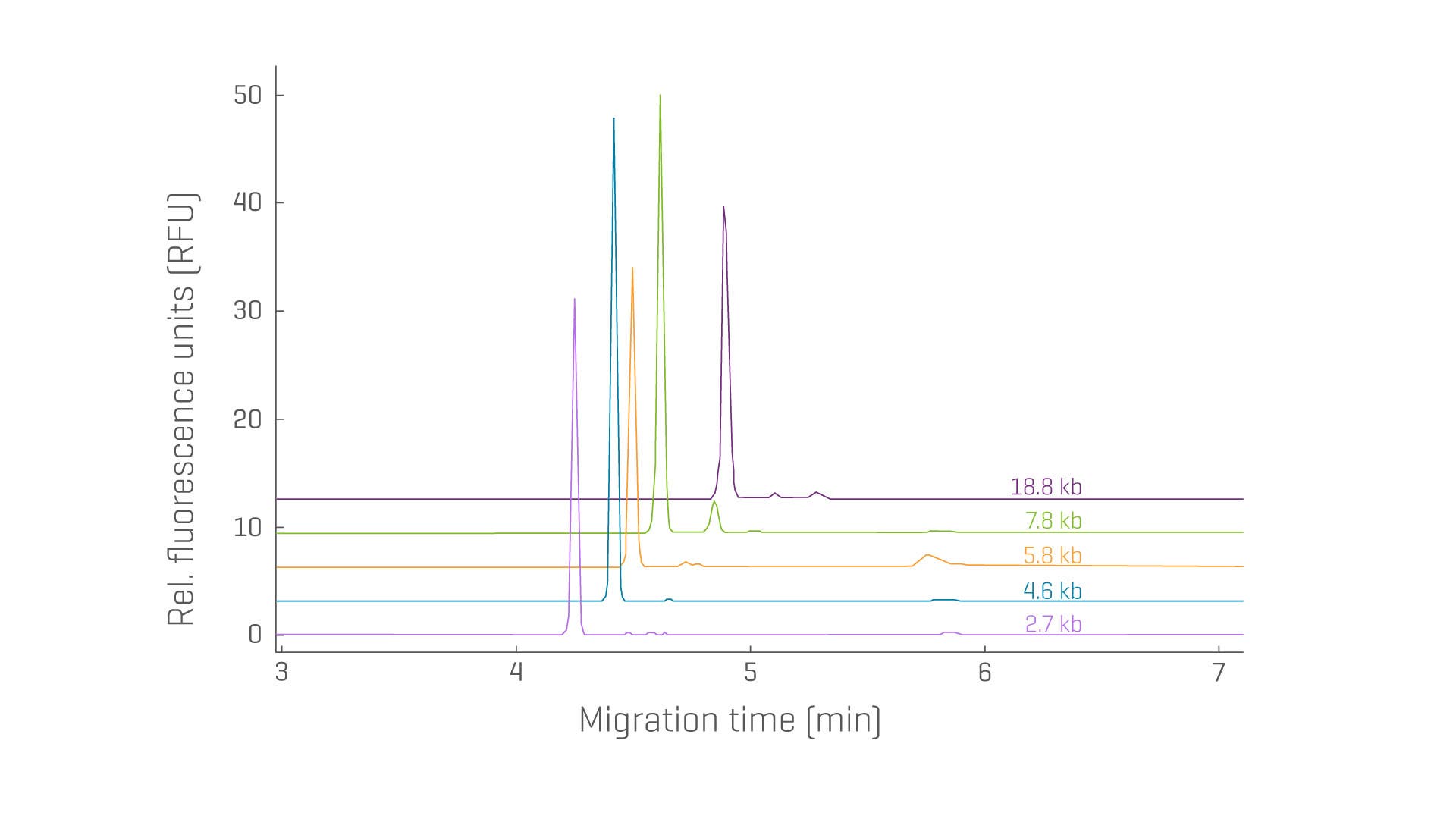
High-purity pDNA is a crucial starting material for manufacturing proteins or viral vectors. Reliable and reproducible separation of topological isoforms for varying pDNA sizes is key to determining the quality.
An efficient transfer of methods from development to quality control (QC) requires highly robust and compliant-ready workflows.
- Take back your time by separating supercoiled isoforms from other isoforms and impurities from 2,000–19,000 base pairs (bp) using one method suitable across a wide size range
- Determine and monitor the purity and stability of supercoiled pDNA, reproducibly
- Streamline your analyses by leveraging pre-built capillary cartridges and ready-to-use gels
- Efficiently transfer methods between the 8-capillary BioPhase 8800 system and the single-capillary PA 800 Plus system without compromising data quality
pDNA isoforms
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 30 cm. (P/N 5080121)
pDNA isoforms
Characterize therapeutic molecules with confidence with the kit-based system.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica capillary cartridge – 30 cm for the PA 800 Plus system.
Linear dsDNA
Workflow
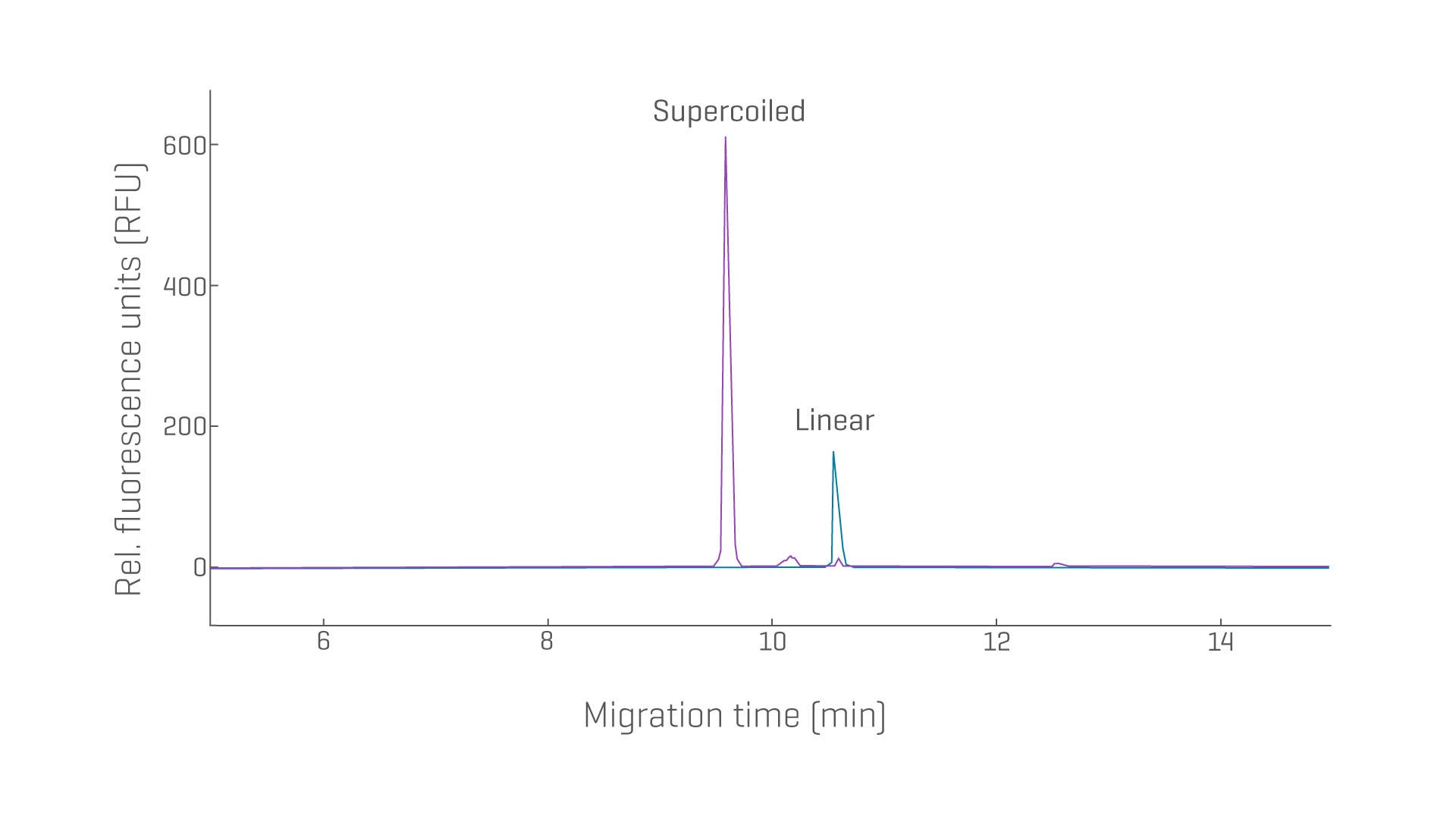
Ensuring the purity of linear dsDNA is of utmost importance for enabling the efficient manufacturing of high-quality messenger RNA (mRNA) and other IVT RNA products, whether the linear DNA is derived from the linearization of pDNA or through restriction enzyme digestion of cell-free DNA.
- Leverage reproducible size determination of linear dsDNA from 100 bp to 20,000 bp to ensure consistent quality for mRNA production
- Streamline your analyses by leveraging pre-built capillary cartridges and increase separation resolution when you need it with pre-built 50 cm capillary cartridges on the BioPhase 8800 system
- Efficiently transfer methods between the 8-capillary BioPhase 8800 system and the single-capillary PA 800 Plus system without compromising data quality
Linear dsDNA
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 30 cm. (P/N 5080121)
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 50 cm. (P/N 5080123)
Linear dsDNA
Characterize therapeutic molecules with confidence with the kit-based system.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica capillary cartridge – 30 cm for the PA 800 Plus system.
pDNA restriction digest
Workflow
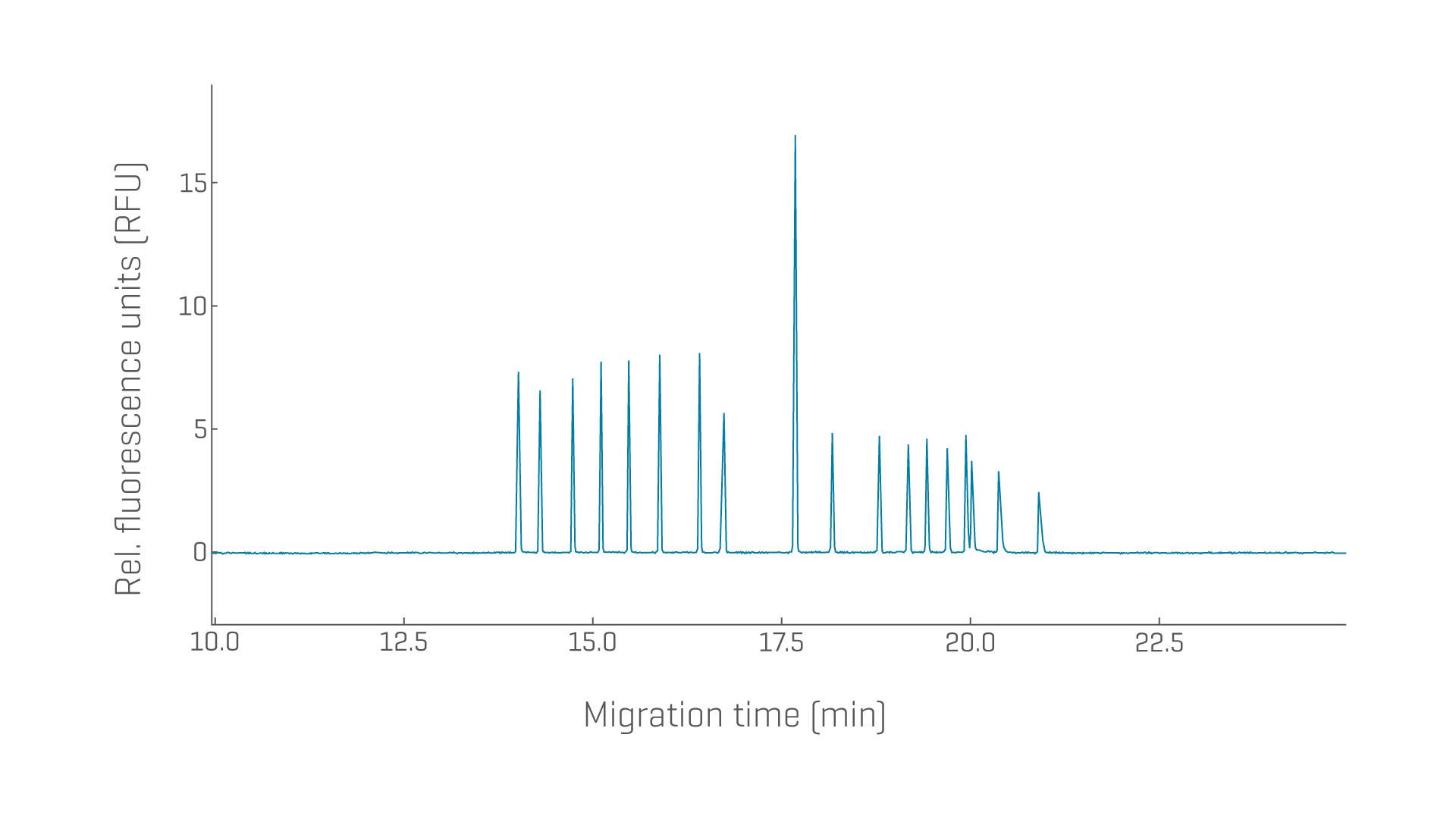
Restriction enzyme digests of pDNA can serve as an identity test and provide size estimation for genes of interest and regulatory or repetitive regions.
- Achieve ultra-high-resolution separation of restriction fragments for plasmid ID, including repetitive sequences
- Leverage excellent size-based separation of linear dsDNA from 100–20,000 bp
- Streamline workflows using pre-assembled 30 cm BFS cartridges
- Enhance resolution with pre-assembled BioPhase BFS cartridges – 8 x 50 cm
pDNA restriction digest
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 30 cm. (P/N 5080121)
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 50 cm. (P/N 5080123)
pDNA restriction digest
Characterize therapeutic molecules with confidence with the kit-based system.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica capillary cartridge – 30 cm for the PA 800 Plus system.
Residual DNA analysis
Workflow
Residual host cell DNA poses a potential safety risk for cell culture-derived vaccines and therapeutic products. Reliable size determination and simultaneous quantitation of residual DNA are crucial to enable risk assessment and ensure product safety.
- Determine risk based on the quantity and size of impurities
- Leverage excellent size-based separation of linear dsDNA from 100–20,000 bp
- Streamline workflows using pre-assembled 30 cm BFS cartridges
- Enhance resolution with pre-assembled BioPhase BFS cartridges – 8 x 50 cm
Residual DNA analysis
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 30 cm. (P/N 5080121)
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 50 cm. (P/N 5080123)
Residual DNA analysis
Characterize therapeutic molecules with confidence with the kit-based system.
Determine the purity and identity of various plasmid constructs with exceptional resolution and reproducibility.
Pre-assembled bare-fused silica capillary cartridge – 30 cm for the PA 800 Plus system.
All resources
-
Technical note
Comprehensive plasmid topology and linear DNA sizing analysis
-
Technical note
Intermediate precision study of DNA analysis
-
Technical note
Seamless method transfer and consistent results across single and multi-capillary systems for plasmid purity analyses
-
Brochure
Consumable and reagents catalog for the BioPhase 8800 and PA 800 Plus systems
Associated applications
Break through barriers and extend the frontiers of messenger RNA (mRNA), self-amplifying RNA (saRNA) and circular RNA (circRNA) development with intuitive analytical solutions. Confidently innovate with high-quality, sensitive and accurate data to assess integrity, purity and critical quality attributes (CQAs)
Tackle the next frontier in protein therapeutics with analytical strategies ignited by collaboration.
Optimize workflows to reliably detect and monitor process-related impurities. Ensure the safety of next-generation viral products with accurate, reproducible sizing of residual DNA and RNA and simultaneous quantitation.
Optimize your processes with solutions that overcome the analytical challenges of viral vector-based drugs. Take control of full and empty ratios and protein and genome profiles. Break down the boundaries of protein and post-translational modification (PTM) characterization with dedicated high-resolution workflows offering unprecedented depth.