Comprehensive charge variant analysis made simple
The Intabio ZT system couples icIEF separation and UV detection with high-resolution mass spectrometry for peak identification.
Cut out risk, cut out information gaps.
Gain multiple pieces of critical data up front.
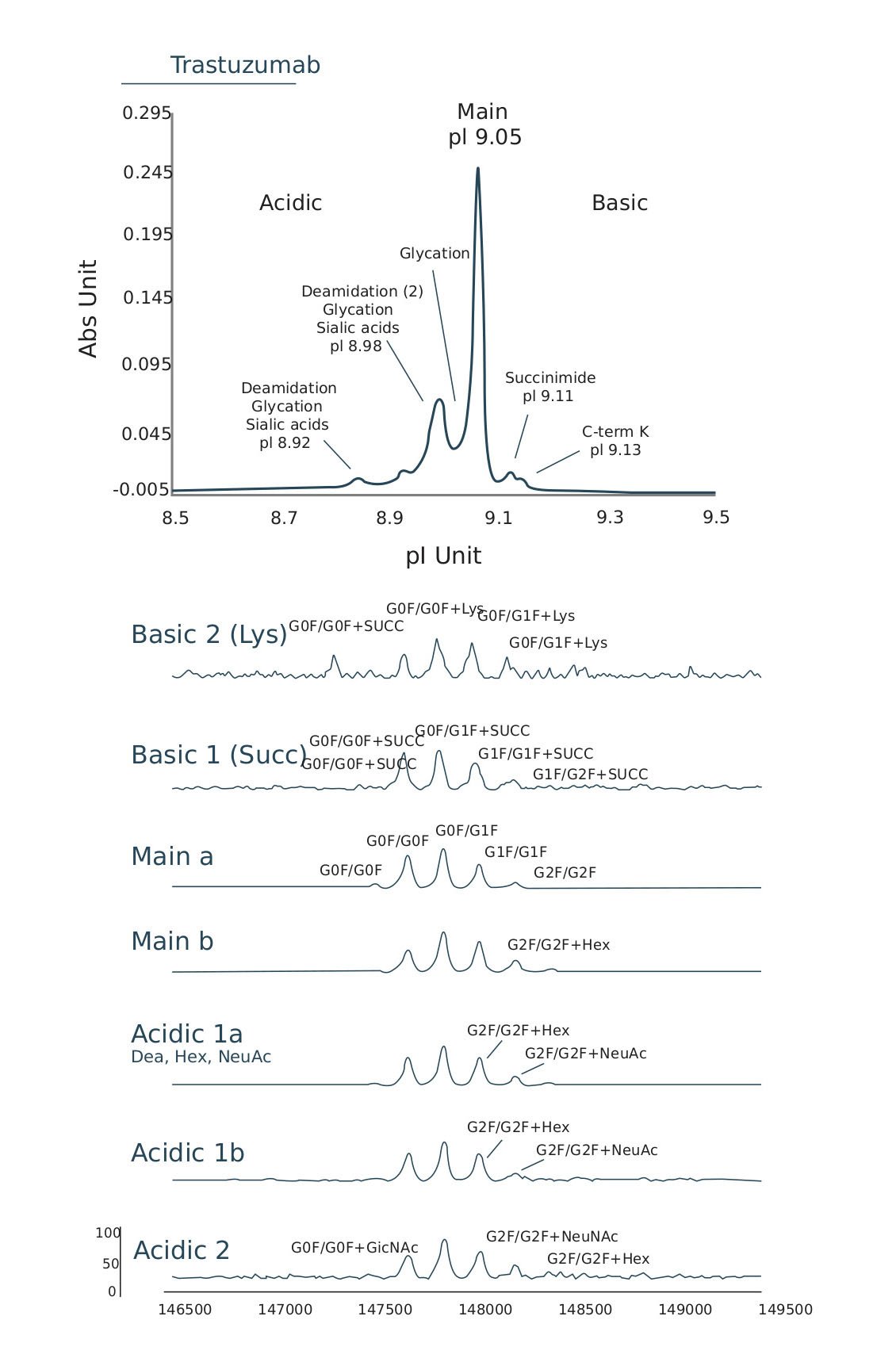
Accelerate candidate selection by achieving separation, quantitation, and direct identification of biopharmaceutical charge variants and their proteoforms with icIEF-UV/MS via the Intabio ZT system.
Comprehensive proteoform identification in minutes
The process of identifying individual charge variant components and interpreting their structural differences conventionally takes weeks and requires the use of multiple instruments, with multiple manual steps.
With the Intabio ZT system - separation, quantitation and identification of individual charge variants can be achieved in minutes on a single platform.
Contact SCIEX
Request more information to learn how the Intabio ZT system can work for specific your needs.
Leverage key analytical functions with microfluidic chip-based technology
The Intabio ZT cartridge enables key analytical functions using proprietary microfluidic chip-based integrated icIEF-UV/MS technology.
- Separation of charge variants with imaged capillary isoelectric focusing (icIEF)
- Quantitation with real-time UV detection
- Electrospray ionization for further mass spectrometry identification
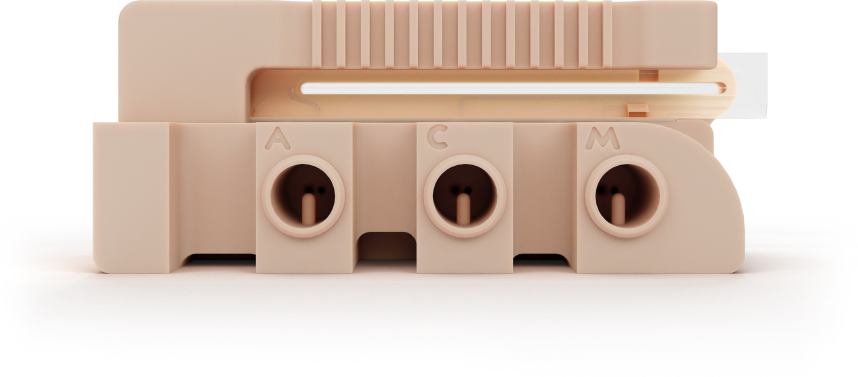
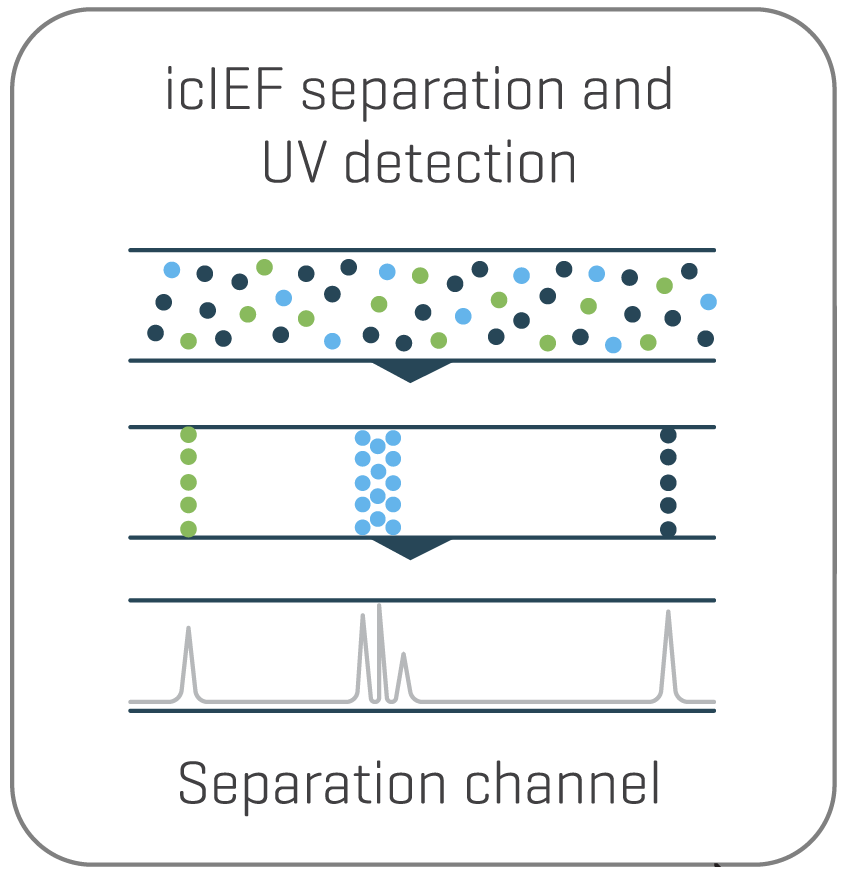
Separation of charge variants with imaged capillary isoelectric focusing (icIEF) and quantitation with imaged UV detection occurs within the separation channel

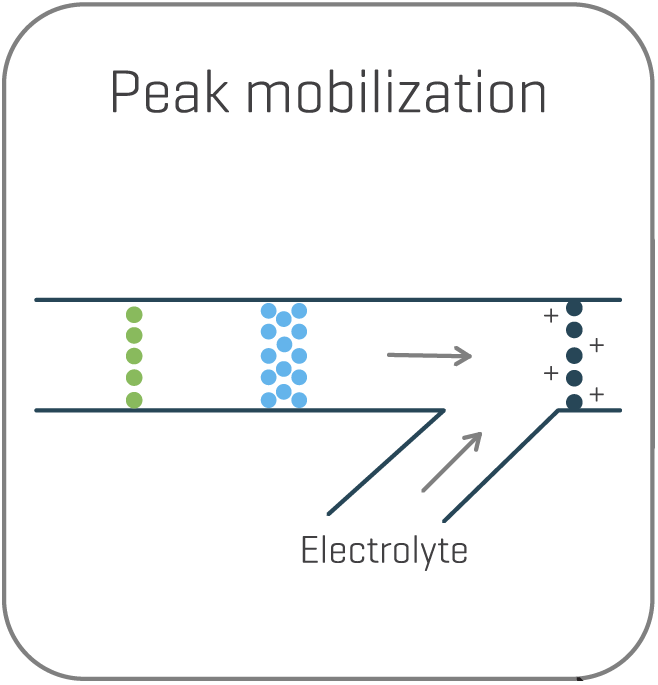
To enable electrospray ionization, electrolyte is introduced through the mobilizer channel near the ESI tip, to re-ionize the charge variants. The electric field is re-oriented to initiate the mobilization of the peaks toward the electrospray “ESI” tip. This novel chemical mobilization process ensures that peak resolution is maintained throughout MS detection.

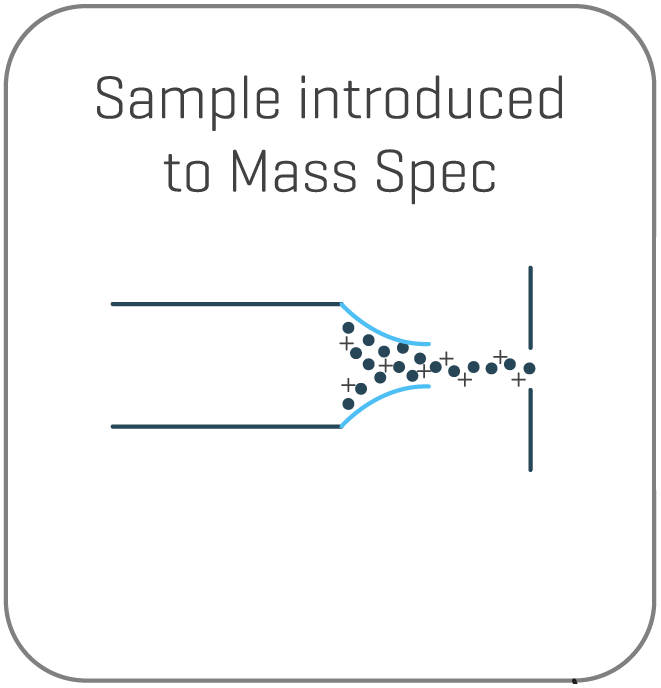
Separated charge variants are introduced to the mass spectrometer by electrospray ionization for peak identification.

Eliminate the guess work with the ZenoTOF 7600 system
Enable a more detailed characterization approach by leveraging the enhanced capabilities of the powerful ZenoTOF 7600 system with EAD fragmentation technology.
Guide decisions with multidimensional analysis using the Intabio ZT system
Let Biologics Explorer software do the heavy lifting
Maintain data continuity with a single workflow producing one, accessible dataset, where UV peaks correlate with MS peaks. Let Biologics Explorer software do the heavy lifting with pre-built workflows for charge variant analysis. Get the required information to make confident decisions, fast.
Contact SCIEX
Request more information to learn how the Intabio ZT system can work for specific your needs.
Separate, characterize, identify
High-throughput product quality characterization with rapid multi-attribute monitoring of intact biotherapeutics and identification of charge variants throughout the drug development pipeline.
-
01:
-
02:
-
03:
Wherever you play in the pipeline, change the game in modern medicine
Intact protein analysis, charge variant analysis, PTM characterization
Charge variants, glycoforms, PQA assessment
Stability/degradation
CQA and PQA monitoring
Associated applications
Featured resources
A 4x increase in sensitivity enables detection and confident identification of challenging proteoforms like deamidation
Take full advantage of advanced characterization capabilities of the EAD enabled ZenoTOF 7600 system with simple conversion from icIEF-UV/MS to EAD based LC/MS.
Gain a level of characterization beyond traditional LC separation and CID based workflows to provide critical information to guide analytical strategies throughout ADC development.
A single platform icIEF-UV/MS workflow on the Intabio ZT system provides confident identification of sialyated glycoproteins.
Designed to provide the confidence you need to achieve your analytical goals.
Facilitate biotherapeutics development with cIEF, icIEF-UV/MS and LC-MS.
Learn about a novel integrated workflow for your charge variant analysis of ADCs.
Highly confident identification of CQAs with an integrated icIEF-UV/MS solution that identifies low abundant PTMs at the intact protein level.
Gain advantages in data quality and time with an integrated system that offers high-resolution and high-throughput separation of intact mAbs and their charge variants.
A single platform icIEF-UV/MS workflow offers high sensitivity and selectivity to detect low-abundant proteoforms that could impact product quality.
Comprehensive characterization and identification of the charge variants of deglycosylated NISTmAb in minutes.
Biopharmaceutical product development is all about product knowledge, whether it be writing a regulatory submission, investigating unexpected results, or performing extended characterization.
Guide decisions during cell line development with charge variant identification at the intact level.