Abstract
This technical note demonstrates the powerful capabilities of an imaged capillary isoelectric focusing (icIEF)-UV/MS workflow for charge heterogeneity analysis of human transferrin (Tf), an acidic glycoprotein detected in an isoelectric point (pI) range of 5.5-6.5. The icIEF-UV/MS workflow provides high-resolution charge separation, sensitive detection and confident identification of the Tf glycoforms and charge variants. The coupling of online icIEF separation with accurate mass measurement eliminates the need for labor- and time-intensive fraction collection followed by offline MS analysis, significantly reducing the time to obtain the results from weeks to hours for routine samples.
Charge heterogeneity analysis of protein therapeutics is important for product quality attributes (PQAs) assessment to ensure the quality, safety and efficacy of drug molecules from the development to quality control. Traditional icIEF workflows can provide reproducible charge variant separation for PQA analysis in different biopharmaceutical settings.1 However, it is challenging for these approaches to identify the proteoforms under each charge-variant peak or elucidate complex charge profiles without the mass information. The icIEF-UV/MS workflow offered by the Intabio ZT system overcomes these challenges by coupling high-resolution icIEF separation with accurate mass measurement.2-8 This powerful workflow has proven to be highly effective for charge heterogeneity analysis of protein therapeutics with a wide pI range of 7.3-9.1.3
In this technical note, the capability of icIEF-UV/MS was extended to sialic acid-containing Tf. The streamlined icIEF-UV/MS workflow provides reproducible charge variant separation and confident identification of the Tf glycoforms containing different numbers of sialic acids and charge variants carrying various post-translational modifications (PTMs) (Figure 1).
Key features of the Intabio ZT system for charge variant analysis
- Reproducible charge variant separation: icIEF-UV offers high resolution and high sensitivity for the separation and detection of low-abundant variants or impurities with a high degree of reproducibility. The separation is maintained after chemical modification prior to ESI-MS analysis.
- Accurate peak assignment: The MS capability offered by the icIEF-UV/MS workflow enables confident assignment of the main peak and charge variants, eliminating the need for fractionation and providing a clear advantage over traditional icIEF-UV approaches for characterizing complex charge profiles.
- Fractionation-free method: The coupling of online icIEF separation with MS detection enables 30-min sample analysis and eliminates the need for time-consuming (days to weeks) fractionation followed by separate MS analysis.
- Platform assays: The streamlined icIEF-UV/MS workflow can be easily implemented for routine and advanced charge heterogeneity analysis of protein therapeutics.
Introduction
Protein therapeutics are highly heterogeneous due to the presence of various glycoforms, degradation products, aggregates and charge variants.9,10 Charge heterogeneity analysis is important for formulation and stability studies, CQA assessment and quality control of these biotherapeutic molecules.1,10 While traditional icIEF approaches provide a rapid separation of charge variants, offering a global view of the charge profile, these techniques cannot reveal the identity of proteoforms or impurities under each variant peak. Additionally, it is challenging to interpret the complex charge profiles for correct peak assignment without the ability to perform accurate mass measurements in conventional icIEF workflows. The icIEF-UV/MS workflow offered by the Intabio ZT system offers a viable solution for high-resolution separation, sensitive detection and confident identification of biotherapeutic charge variants.2-8
Tf is an acidic N-linked glycoprotein (pI 5.5-6.5) carrying different numbers of sialic acids.11 This protein serves as an important biomarker for screening individuals with chronic alcohol abuse.11 The main glycoform of Tf consists of 4 sialic acids on 2 Asn sites (tetrasialo). Other Tf glycoforms in lower abundances include disialo, trisialo and pentasialo.
The powerful capabilities of the icIEF-UV/MS workflow for charge variant analysis have been described previously for protein therapeutics with pIs of ~7-9.3 In this work, the ability of the icIEF-UV/MS workflow to characterize acidic proteins such as Tf will be presented.
Methods
Sample preparation: apo-Tf (Sigma Aldrich T1147) was reconstituted with DI water and then mixed with the master mix solution containing 10 mM arginine, 3% Pharmalyte 5 to 8 (Cytiva), 1% Pharmalyte 3 to 10 and 6.0 µg/mL peptide pI markers. The solution was vortexed and degassed by centrifugation.
icIEF-UV/MS: The Tf sample was separated with an Intabio cartridge (SCIEX) installed on the Intabio ZT system (SCIEX). UV absorbance measurements were collected at 1 Hz during the focusing and mobilization steps. The samples were introduced into the ZenoTOF 7600 system by a metered 3 µL/min flow of chemical mobilizer, and the data was acquired.
Data analysis: UV traces and mass spectra from icIEF-UV/MS analysis were interpreted using Biologics Explorer software (SCIEX). Each peak in the charge profile was integrated using the Intabio software to determine its peak area and percentage composition. Intact masses of the main peak and charge variants were determined from the deconvolution of the corresponding mass spectra.
High-resolution separation and detection of Tf charge variants using icIEF-UV
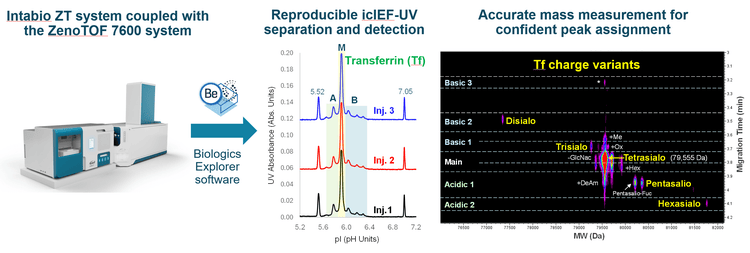
It was demonstrated previously that the Intabio ZT system offers a high-resolution separation of biotherapeutic charge variants in a reproducible manner.2-8 Figure 1A shows the icIEF-UV profiles from 3 consecutive injections of Tf. The high-resolution separation of the main species (M) and charge variants (A and B) was maintained between injections. These species were detected in a pI range of 5.5-6.5, with the main species observed at a pI of ~5.9. The pIs of these species remained consistent in triplicate injections (Figure 1A). The reproducibility in charge variant separation and the consistency in pI measurement is important for the implementation of the icIEF-UV/MS workflow in different biopharmaceutical settings.
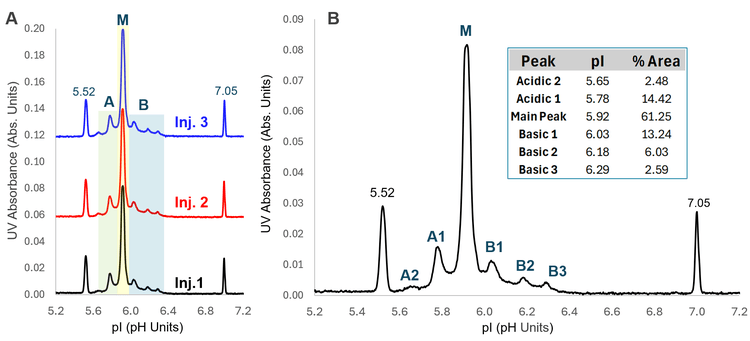
Figure 2B shows a closer view of an icIEF-UV profile where each charge variant peak of Tf is labeled (A1-A2 and B1-B3). The relative abundances of the main peak and charge variants calculated from the icIEF-UV/MS data are provided in the table in Figure 2B. The percent composition of the main Tf glycoform is ~61%, while the acidic and basic variants were calculated to be ~17% and ~22%, respectively. Accurate mass measurement was performed to identify the proteoforms under each of the variant peaks detected in the icIEF-UV profile, as described below.
Confident assignment of Tf glycoforms and charge variants using icIEF-UV/MS
The Intabio ZT system enables simultaneous separation, detection, identification and quantitation of charge variants in a single assay by integrating charge separation and UV detection offered by icIEF-UV with the accurate mass measurement capability of the ZenoTOF 7600 system.2-8 The icIEF-UV/MS data is interpreted using an optimized intact MS workflow template within Biologics Explorer software, which also provides powerful tools for results review and visualization.
Figure 3 shows an ion map from Biologics Explorer software with the assignment of the Tf glycoforms and charge variants detected by icIEF-UV/MS. The measured mass of the main species is 79,555 Da, corresponding to the tetrasialo Tf with 1 fucose moiety (Fuc). The decrease in sialic acids in disialo and trisialo Tf led to the detection of these 2 species on the lower pI (basic) side of the main species (Basic 1 and 2 in Figure 3). In contrast, the increasing number of sialic acids in pentasialo and hexasialo Tf resulted in their detection at lower pIs (Acidic 1 and 2 in Figure 3). These results demonstrate the capability of icIEF-UV/MS for the charge separation and intact MS analysis of sialylated glycoproteins, which are often challenging to characterize using traditional LC-MS approaches.
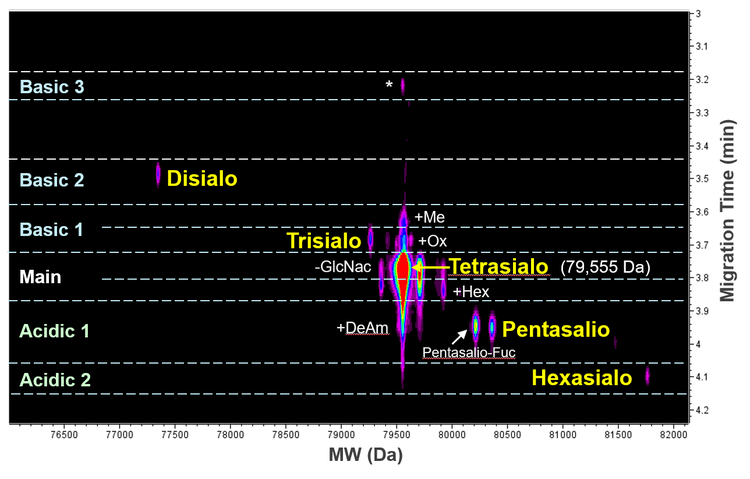
The icIEF-UV/MS workflow also led to the separation and identification of low-abundant Tf charge variants carrying various modifications, including deamidation, oxidation, glycation and methylation (Figure 3). A charge variant detected in Basic 3 (labeled with “*” in Figure 3) has a similar measured mass as the main peak and is potentially a mutant species (e.g., D→N), which was reported for Tf in the literature.12 Further peptide mapping experiments will be performed to confirm the identity of this species. These results demonstrate the advantages of the icIEF-UV/MS workflow for routine to advanced charge heterogeneity analysis.
In summary, the icIEF-UV/MS workflow can be a valuable tool for the rapid monitoring and characterization of proteins with a wide range of pI, including acidic proteins carrying sialic acids, accelerating product development and quality assessment.
Conclusion
- The icIEF-UV/MS workflow enables direct coupling of icIEF analysis, UV quantitation and high-resolution mass spectrometry measurements for charge variant peak identification and comprehensive charge heterogeneity analysis of proteins
- This powerful workflow provides for high-resolution separation, sensitive detection and confident identification of charge variants for proteins with a wide range of pI, including acidic proteins (pI<7)
- The icIEF-UV/MS analysis revealed that the main glycoform of Tf is the tetrasialo, while the di-/tri-sialo and penta-/hexa-sialo forms were identified in the basic and acidic variants, respectively
- icIEF-UV/MS also identified low-abundant charge variants containing various PTMs, including deamidation, oxidation, glycation and methylation
- The icIEF-UV/MS enabled the separation and identification of proteoforms under each charge variant peak without the need for offline fractionation, saving time and cost during product development and quality assessment
References
- Jiaqi Wu et al. (2022) Imaged capillary isoelectric focusing: Applications in the pharmaceutical industry and recent innovations of the technology. Trends in Anal Chem. 150:116567.
- Ostrowski M. Rapid multi-attribute characterization of intact bispecific antibodies by a microfluidic chip-based integrated icIEFMS technology. Electrophoresis. 2022 Oct;1-9.
- Direct and rapid multi-attribute monitoring of multiple intact monoclonal antibodies with a wide isoelectric-point range of 7.3 to 9.1. SCIEX technical note, MKT-26996-A.
- N-linked glycan analysis of NISTmAb, etanercept and trastuzumab. SCIEX technical note, MKT-27527-A.
- A comprehensive workflow to characterize deglycosylated NISTmAb using imaged capillary isoelectric focusing (icIEF)-UV/MS. SCIEX technical note, MKT-27945-A.
- In-depth charge heterogeneity analysis of antibody-drug conjugates with a streamlined icIEF-UV/MS and EAD-based peptide mapping workflow. SCIEX technical note, MKT-32353-A.
- Charge variant analysis of antibody-drug conjugates using an icIEFUV/MS workflow. SCIEX technical note, MKT-29837-A.
- Leverage orthogonal technologies: cIEF, icIEF-UV/MS and LC-MS to facilitate biotherapeutics development. SCIEX technical note, MKT31116-A.
- Anna Robotham and John Kelly. (2020) LC-MS characterization of antibody-based therapeutics: recent highlights and future prospects. Approaches to the Purification, Analysis and Characterization of Antibody-Based Therapeutics. Chapter 1:1-33.
- Yi Du et al. (2012) Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MABS. 4(5):578- 585.
- Alexandre Raynor et al. (2024) Chapter One - Biochemical diagnosis of congenital disorders of glycosylation. Advances in Clin. Chem. 110:1-43.
- A.S. Knisely et al. (2004) Molecular characterization of a third case of human atransferrinemia. Blood 104(8):2607.