Capillary electrophoresis for biopharma drug development

Unlock the future with multiple characterization assays on one platform
Capillary electrophoresis (CE) on the PA 800 Plus system enables ultra-high quality separation day in and day out for the development of proteins and next generation therapeutics.
Achieve a better understanding of protein purity, nucleic acid purity and integrity, protein charge heterogeneity, capsid protein purity, and glycan distribution. Overcome the challenges of an ever-changing pipeline with a single platform designed to minimize method development and maximize innovation.
The assessment of your biopharmaceutical deserves the trusted expertise of SCIEX CE.
Benefits
Flexibility
Gain valuable insights into quality attributes of biotherapeutics with kit-based assays and customizable features to address the evolving drug development pipeline.
Quality
Achieve the resolution and reproducibility you need using industry and regulatory trusted technology, and meet data management needs with platform compatibility.
Portability
Develop and lock down methods applicable to QC or regulated environments. Easily transfer methods between instruments or sites using robust kit-based workflows with proven portability.
Key features
Liquid temperature control
Nail consistent outcomes
Even the slightest temperature variations can throw off your results. Exclusive recirculating liquid coolant enables the precise control of separation temperature in the capillary.
Multiple injection modes
Flexibility is everything.
Ensure consistent, reproducible results with precise control. Get top-notch resolution with electrokinetic injection, tackle sample ionic strength with hydrodynamic injection, and master sensitivity with field-amplified sample stacking.
Separation voltage
When more than just speed matters.
Strike the perfect balance between resolution and analysis time. Harness up to 30 kV separation voltage to tailor for your workflow and sample needs.
Detection wavelength
Cut out the noise and irrelevant targets.
Boost the signal-to-noise ratio for specific analytes and ensure spot-on, reliable results by adjusting a wide range of detection wavelengths.
Capillary length
Switch up the size to boost separation.
Enhance selectivity for specific analytes and achieve superior resolution by adjusting the capillary length and choosing the right capillary coating.
Detector type
Not everything is easy to spot.
Achieve high sensitivity or detect lower concentrations by switching between UV and LIF detectors based on your needs.
Discover kit-based workflows that support core industry assays
Monoclonal antibody and complex protein applications
- Protein purity and heterogeneity
- Charge heterogeneity monitoring
- Charge variant characterization
- N-linked glycan analysis
Cell and gene therapy applications
- Viral vector capsid protein purity
- RNA purity and integrity for small and large species
- Plasmid isoform and linear double-stranded DNA (dsDNA) analysis
- Empty vs. full adeno-associated virus (AAV) analysis
- Single-stranded oligonucleotide analysis
mAb and mAb variants
Purity and heterogeneity analysis with CE-SDS
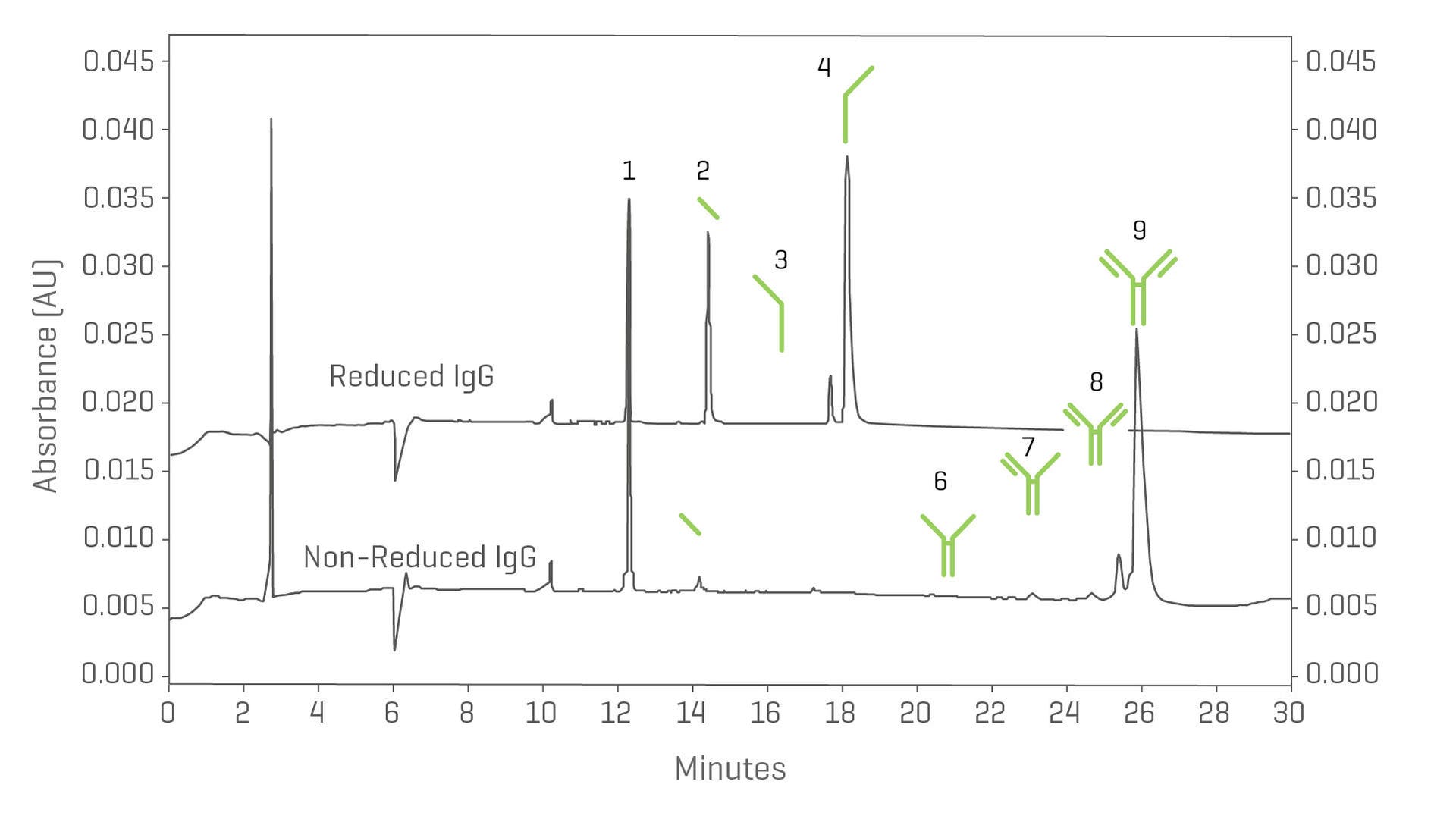
Determine the apparent molecular weight of proteins to evaluate the size heterogeneity, purity and manufacturing consistency of biologics with capillary electrophoresis-sodium dodecyl sulfate (CE-SDS).
Obtain automated, quantitative purity data on both intact and reduced mAbs with speed and accuracy.
- Fastest CE-SDS separation time (12–18 minutes)
- Quantitation of impurities down to 0.01%
Analysis of both reduced (top) and non-reduced (bottom) IgG suitability standard.
Peak identification: 1: Internal standard (10 kDa); 2: Light chain (L); 3: Non-glycosylated (NG) Heavy chain (H); 4: Heavy chain (H); 6: Heavy chain (2H); 7: 2 heavy 1 light chain (2H1L); 8: NG HC; 9: IgG monomer.
Native state charge variant analysis with CZE
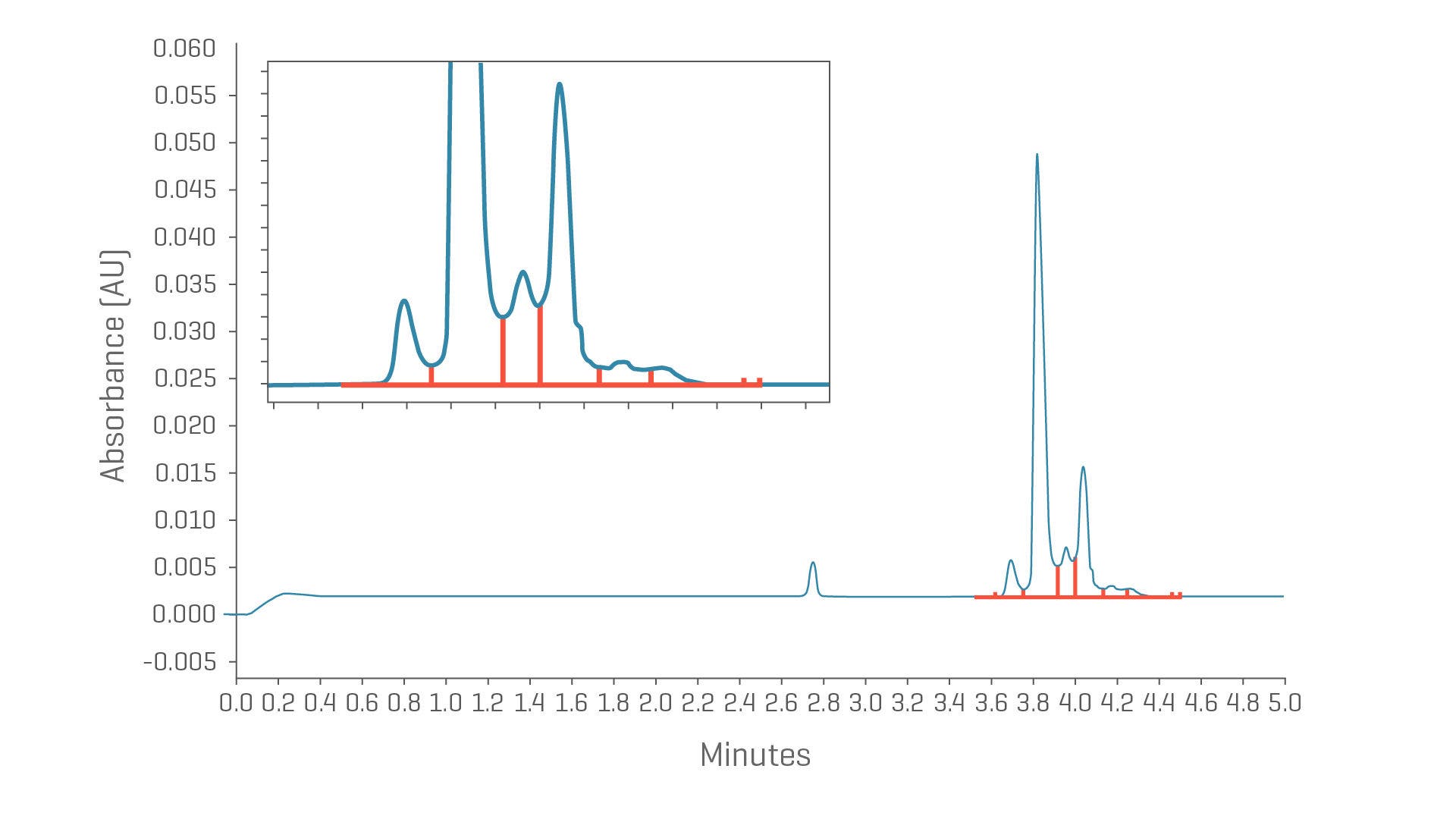
Capillary zone electrophoresis (CZE) combines the benefits of native state analysis with the speed and resolution expected of a capillary electrophoresis method. CZE can be easily applied to therapeutic proteins such as antibody-drug conjugates (ADCs), pre-clinical monoclonal antibodies (mAbs), and fusion proteins with little to no method development.
Simply dilute, shoot and prepare for your next CE application.
- Screen charge variants in 10 minutes
- Save time with fast, straightforward sample preparation
- Apply the same method to a wide pI range
Full view and zoomed electropherogram of trastuzumab.
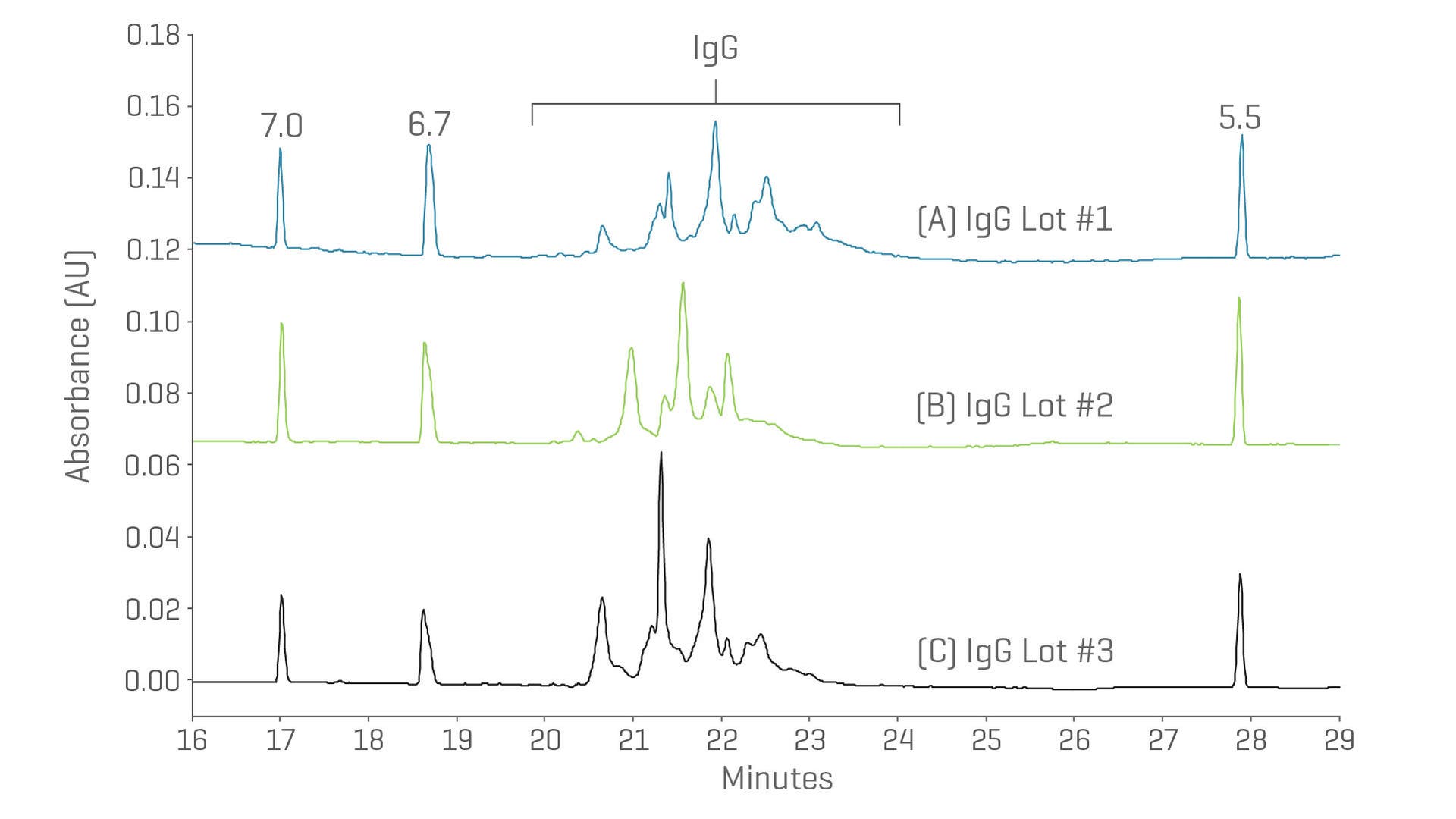
Protein charge heterogeneity with ultra-high resolution cIEF
Establish the identity and stability of the molecule with accurate determination of a protein’s charge heterogeneity using capillary isoelectric focusing (cIEF).
- Gain isoelectric point range and accuracy in 1 run
- High pI accuracy on extreme acidic and basic variants
- High resolution throughout the pI range
cIEF separation of different commercial lots under identical separation conditions.
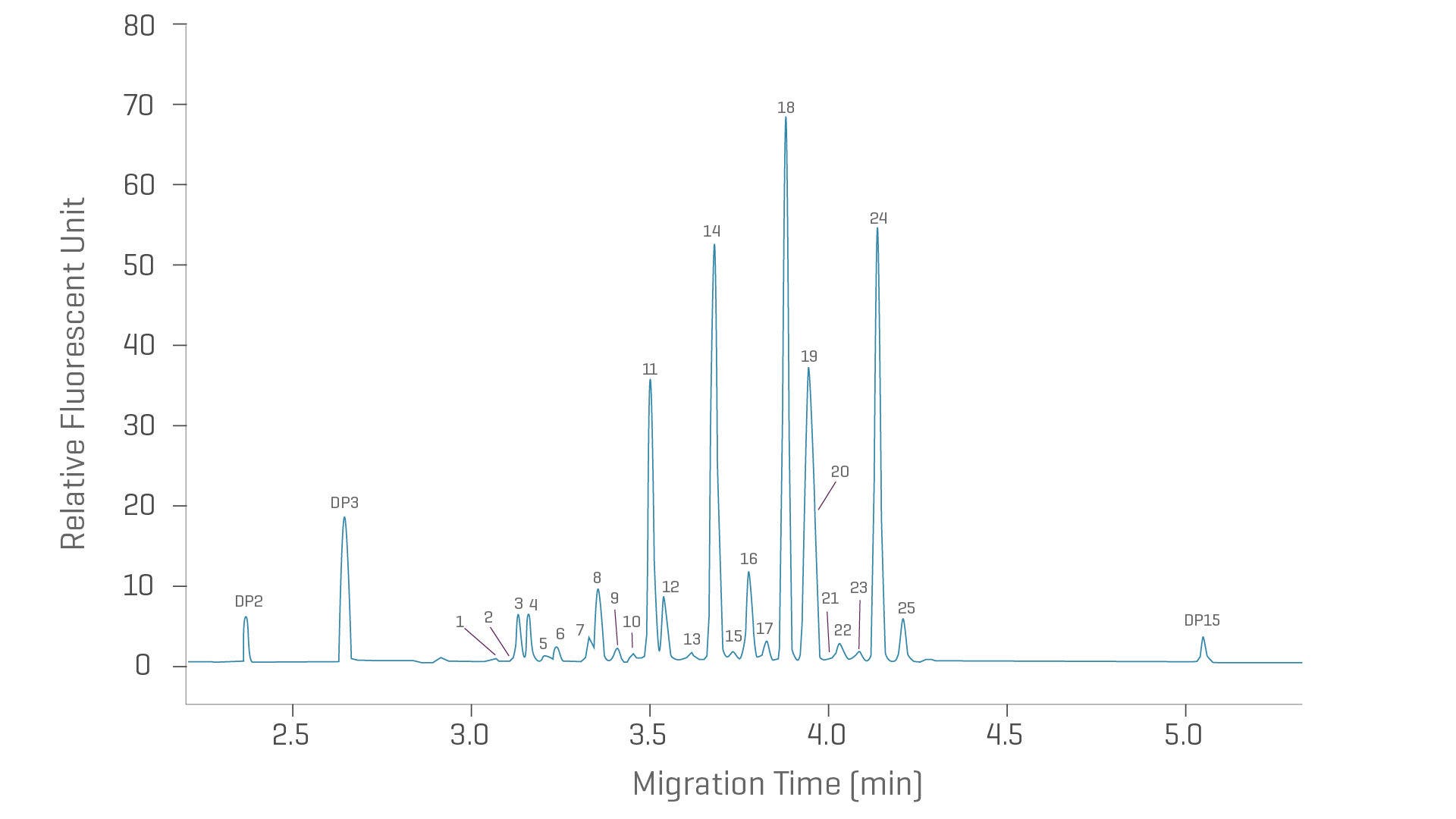
Rapid glycan identification
The fast glycan labeling and analysis kit delivers rapid glycan heterogeneity identification. Combine simplified sample preparation, rapid separations, and automated glycan identification to make confident decisions quickly.
- 1-hour sample prep based on magnetic bead technology
- Up to 2x faster than HILIC
- Automated glycan ID
- Run 1 - 96 samples at a time
Ultrafast high resolution CE analysis of PNGase F released and fluorophore labeled IgG N-glycans using the HR-NCHO gel buffer.
In vitro transcribed RNA
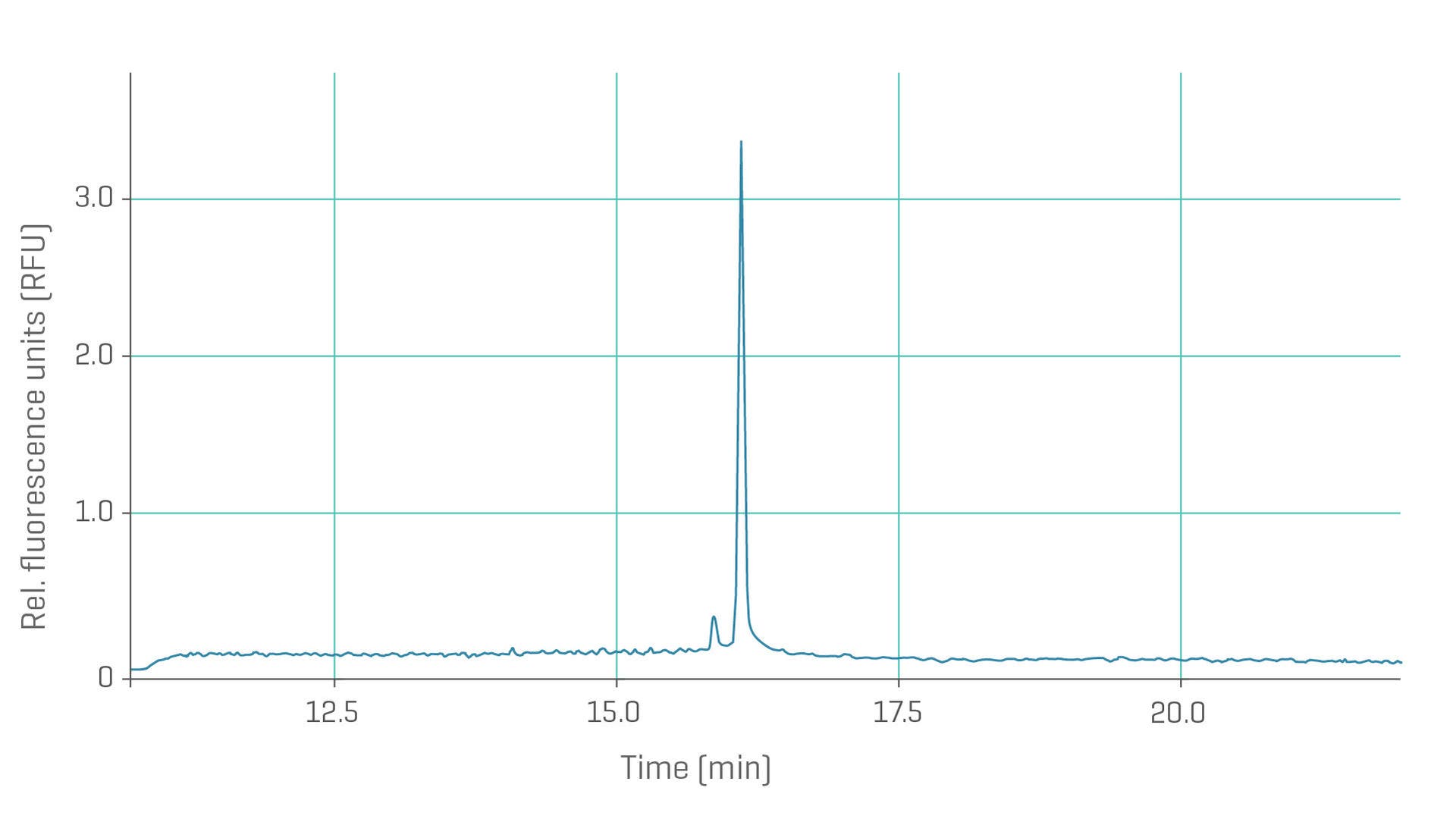
Purity and integrity of mRNA and other IVT RNA with CGE
RNA integrity and quality analysis is critical to ensuring the efficacy of RNA-based therapeutics and vaccines. Designed for the separation of single-stranded oligonucleotides from 50-9,000 nucleotides and beyond.
- Conduct analyses from early development to QC
- High resolution for LNP release mRNA fragments and CRISPR Cas9 therapeutic systems
Electropherogram of mRNA extracted from an LNP.
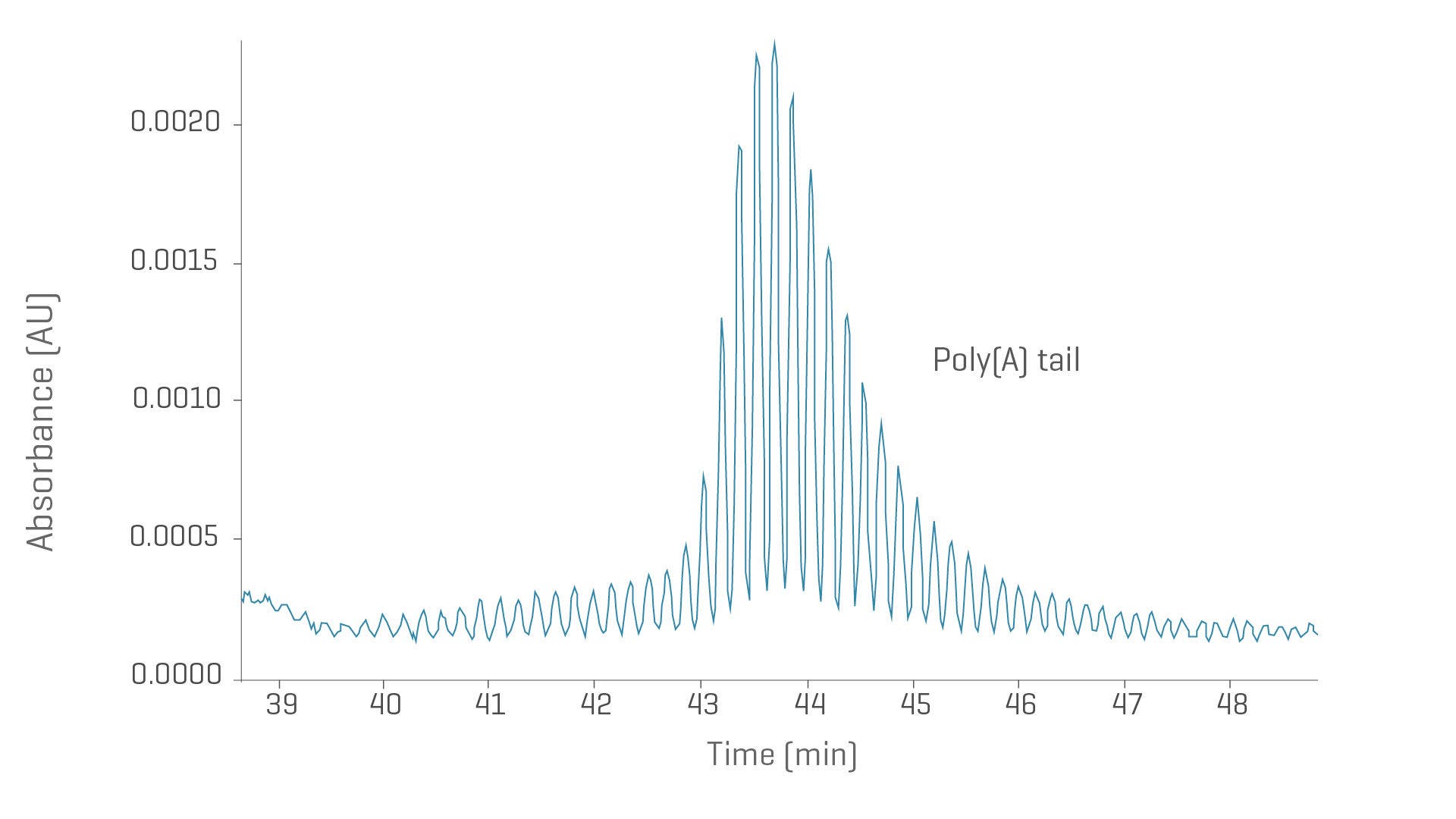
3’ poly(A) tail size assessment
Understanding product stability lays the foundation for improved drugs. Give your team the power to determine the lengths of the adenosine tails and their distribution profiles in mature mRNA products with the ssDNA 100-R kit.
- Obtain size information for 3’ tails with excellent base resolution
- Analyze single-stranded DNA with linearity from 10 to 100 bases
Poly[A] tail analysis from mRNA with CGE-UV. Electropherogram shows single-base resolution of mRNA poly[A] tails with most abundant species of 121 nt in length.
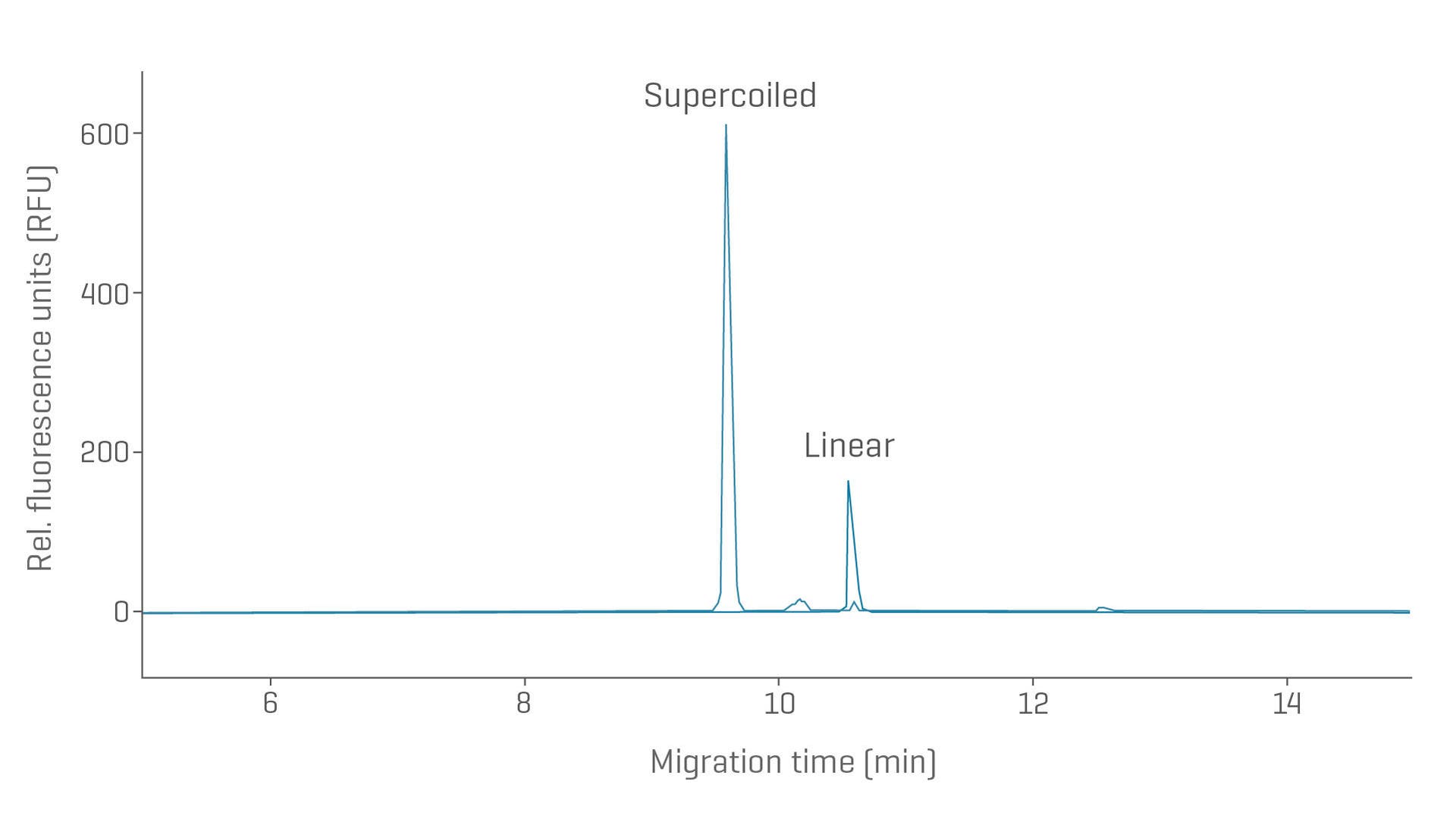
Purity and size of critical starting material with CGE
The quality of pDNA is crucial for high-efficiency manufacturing of proteins, viral vectors, and in vitro transcribed RNA (IVT RNA). Determine linearization efficiency with excellent separation for different topological variants of pDNA.
- Wide size range 100 bp to 20,000 bp
- Quantitative
- Confidently transfer assays from development to QC
Linearized plasmid purity analysis and size estimation of a 7.9kb plasmid sample. The electropherogram shows the sample prior to linearization, containing mainly the supercoiled isoform and after linearization.
Viral vectors
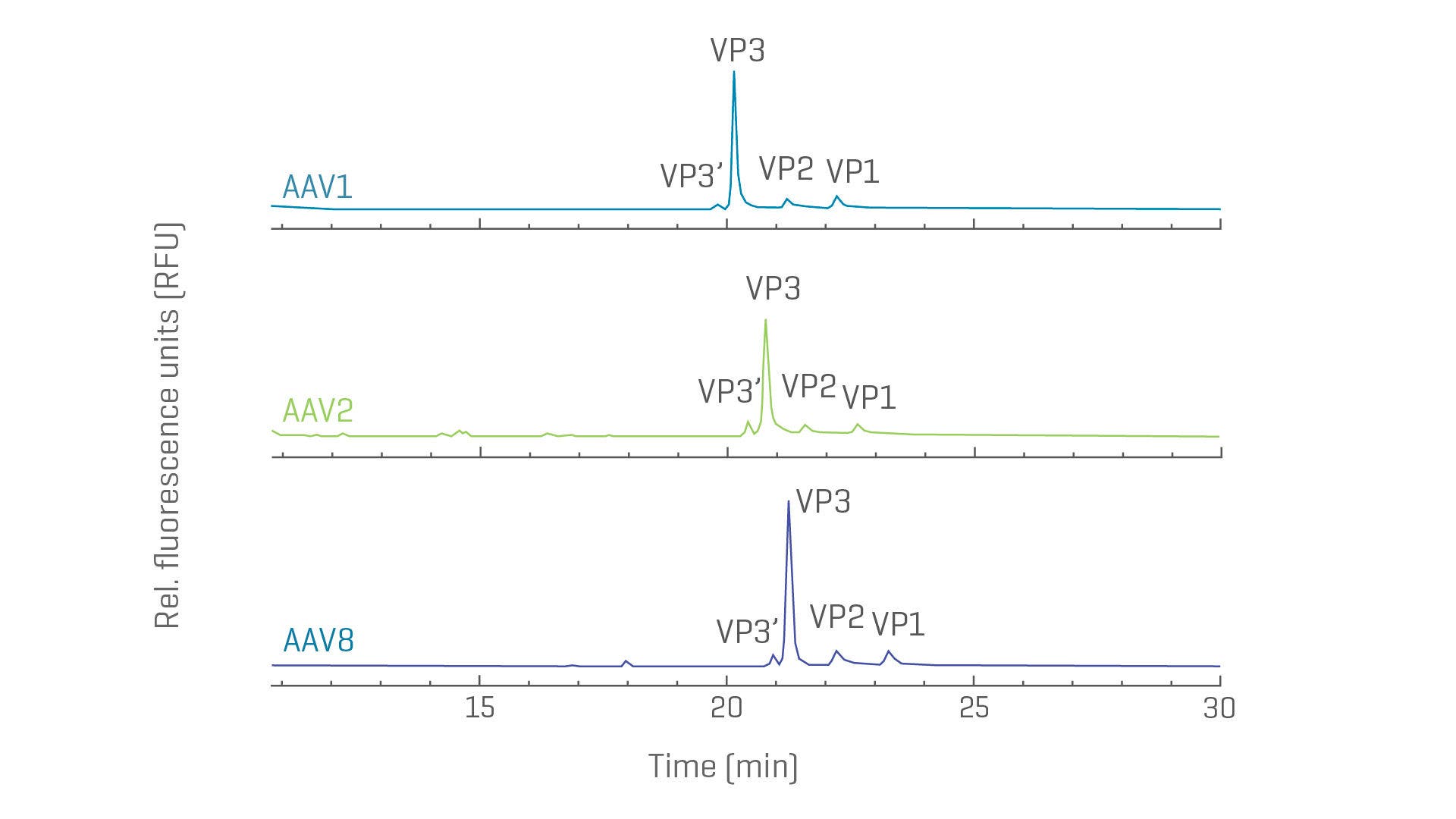
Capsid protein purity and ratios with CE-SDS
Viral vector characterization for vaccine and therapeutic drug development includes assessing the viral proteins. While information on purity and protein ratios can be obtained with liquid chromatography-based methods, an orthogonal approach with CE is advantageous to avoid missing VP’ forms. Avoid lengthy assay adjustments with a kit-based protein profiling workflow suitable across serotypes and viral vectors.
- Determine protein-based titer with confidence
- Understand protein profiles and impurities using high separation power
- Reclaim your time with faster method development and the ability to run larger sample batches
Separation of VP proteins from different AAV serotypes. Excellent resolution of VP proteins labelled with fluorescence dye was acheived with CE-SDS-LIF.
Virus genome integrity with CGE
The integrity and purity of the viral genome are CQAs that impact vector potency, immunogenicity and transduction efficiency. Simplify viral vector genome analysis with a workflow suitable across serotypes and viral vectors.
- Confidently determine genome integrity, genome titer, and impurities
- Run high-quality analyses smoothly and reproducibly with a kit-based turnkey solution
- Streamline data management through compatibility with data management systems
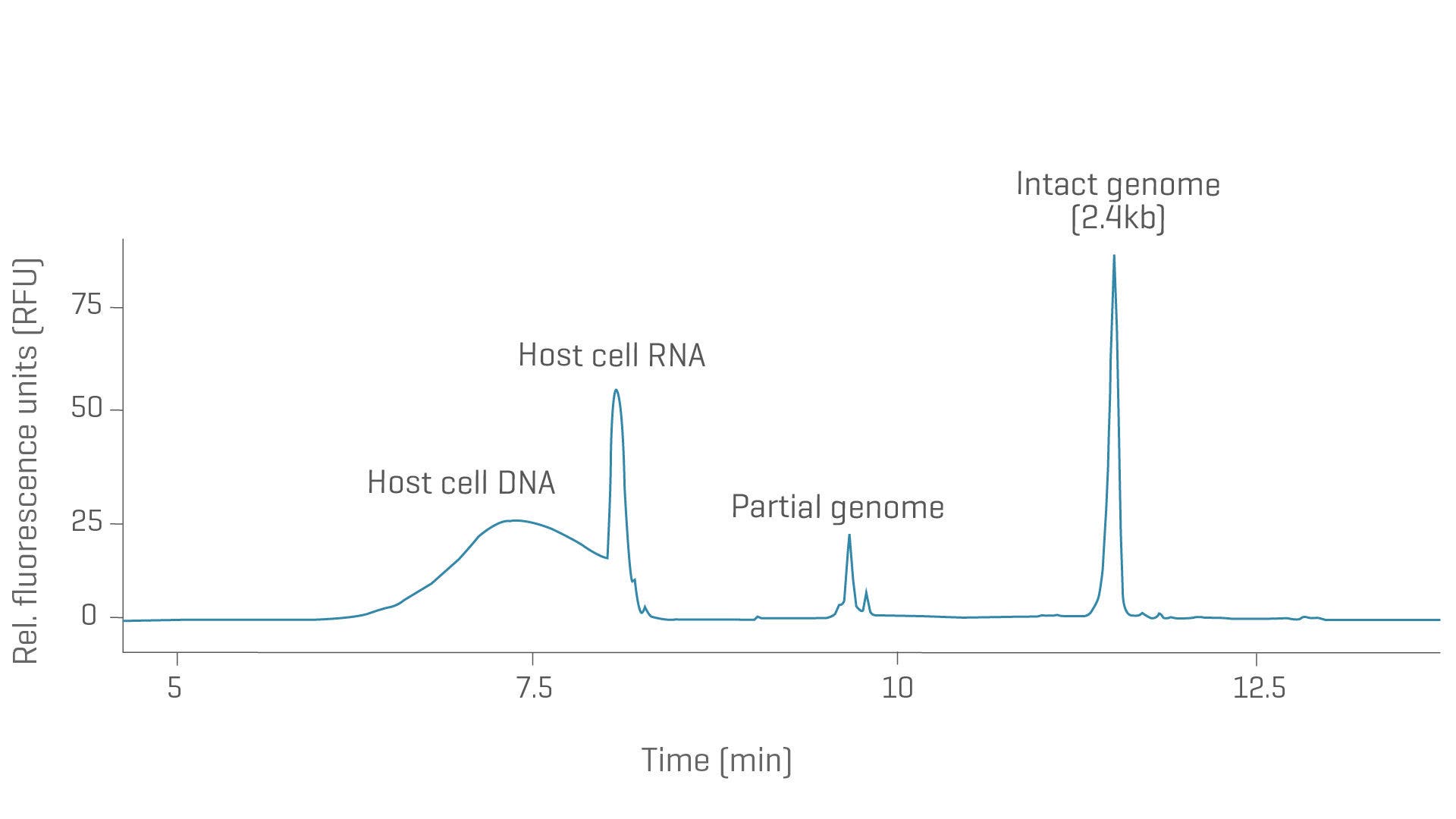
High-resolution genome integrity analysis of AAV8 with CGE and LIF detection. The intact genome of 2.4kb was well separated from potential partial genomes and host cell RNA and DNA impurities.
Empty full capsid ratio with CGE and CE-SDS
Determine multiple CQAs, including full-and-empty ratios, on one platform.
- Analyze genome integrity, capsid proteins, and full-and-empty ratios, including partial capsids, with serotype-independent workflows
- Cover your compliance needs through compatibility with common data management systems
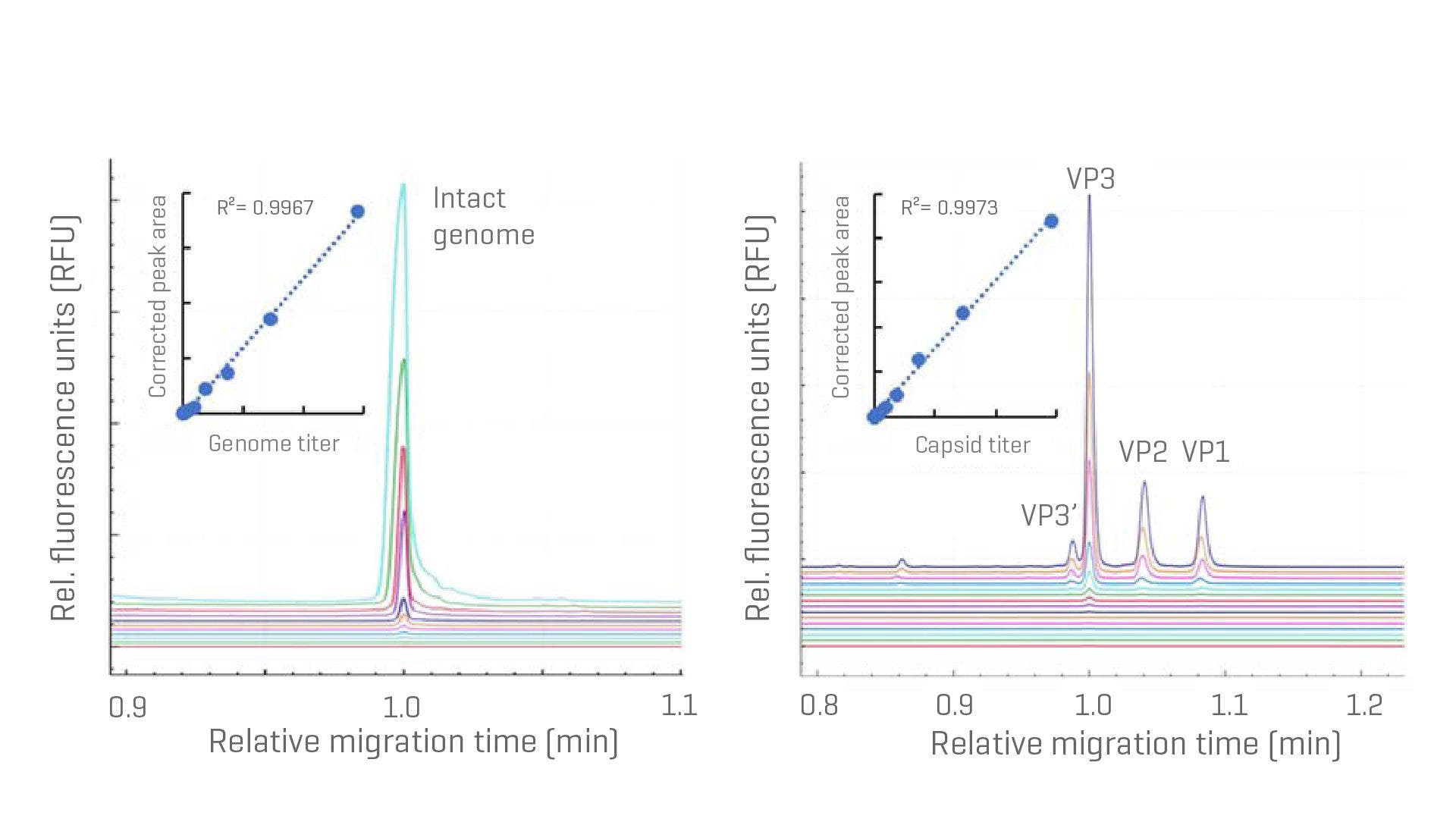
Standard curves for full-and-empty capsid determination. Left: AAV genome titer determination with CGE. The linear dynamic range (LDR) was determined from 2.56x1010 GC/mL-2.62x1013 GC/mL with R² = 0.9967. Right: AAV capsid titer determination with LDR from 6.41x109 GC/mL-2.62x1013 GC/mL with R2 = 0.9973.
Assess critical starting material with CGE
Determine pDNA fragment sizes across a wide range.
- Differentiate topological variants of plasmids and determine purity with ease
- Achieve high sensitivity for early-stage development samples with LIF detection
- Confidently transfer assays from development to QC with excellent precision
- Streamline data management through compatibility with data management systems
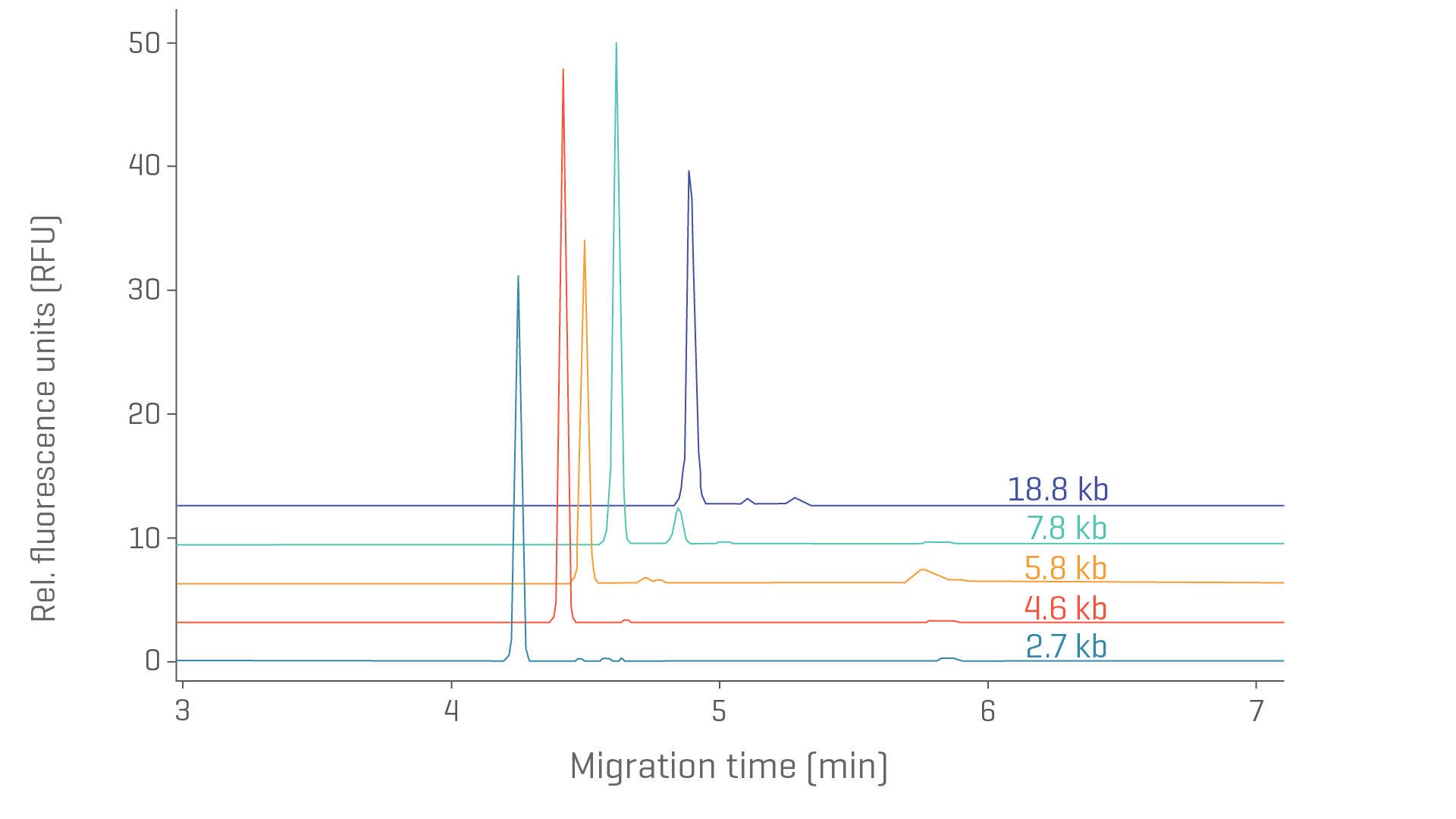
Separation of topological isoforms of 5 plasmids (2.7–18.9 kb) using the DNA 20 kb Plasmid and Linear kit.
Residual host cell DNA with CGE
Assess residual nucleic acid sizes and quantities simultaneously.
- Estimate size and amounts of residual host cell nucleic acids
- Achieve high sensitivity for early-stage development samples with LIF detection
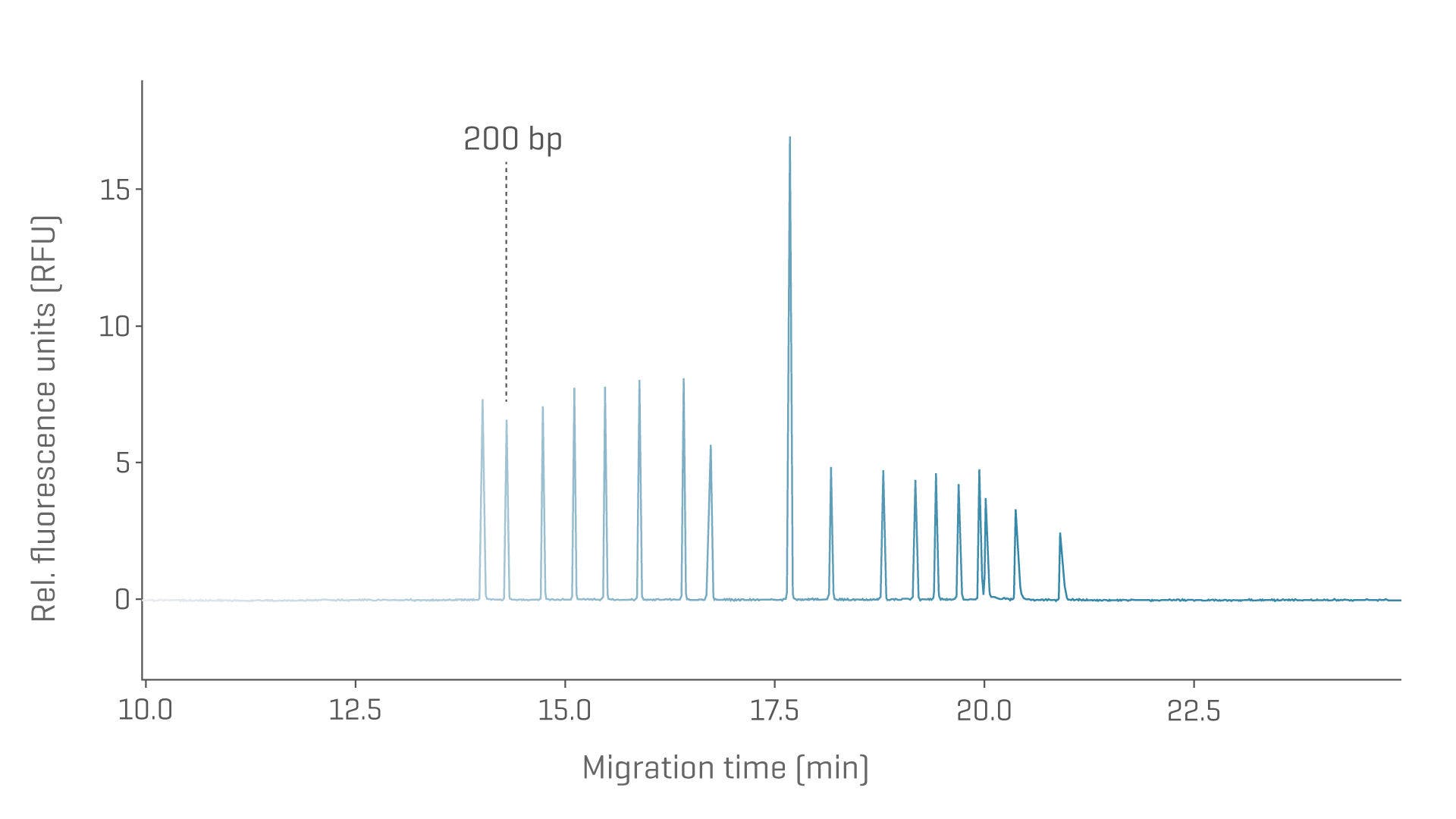
Electropherogram showing baseline separation of a linear dsDNA ladder from 100-15,000 bp.
Gene editing
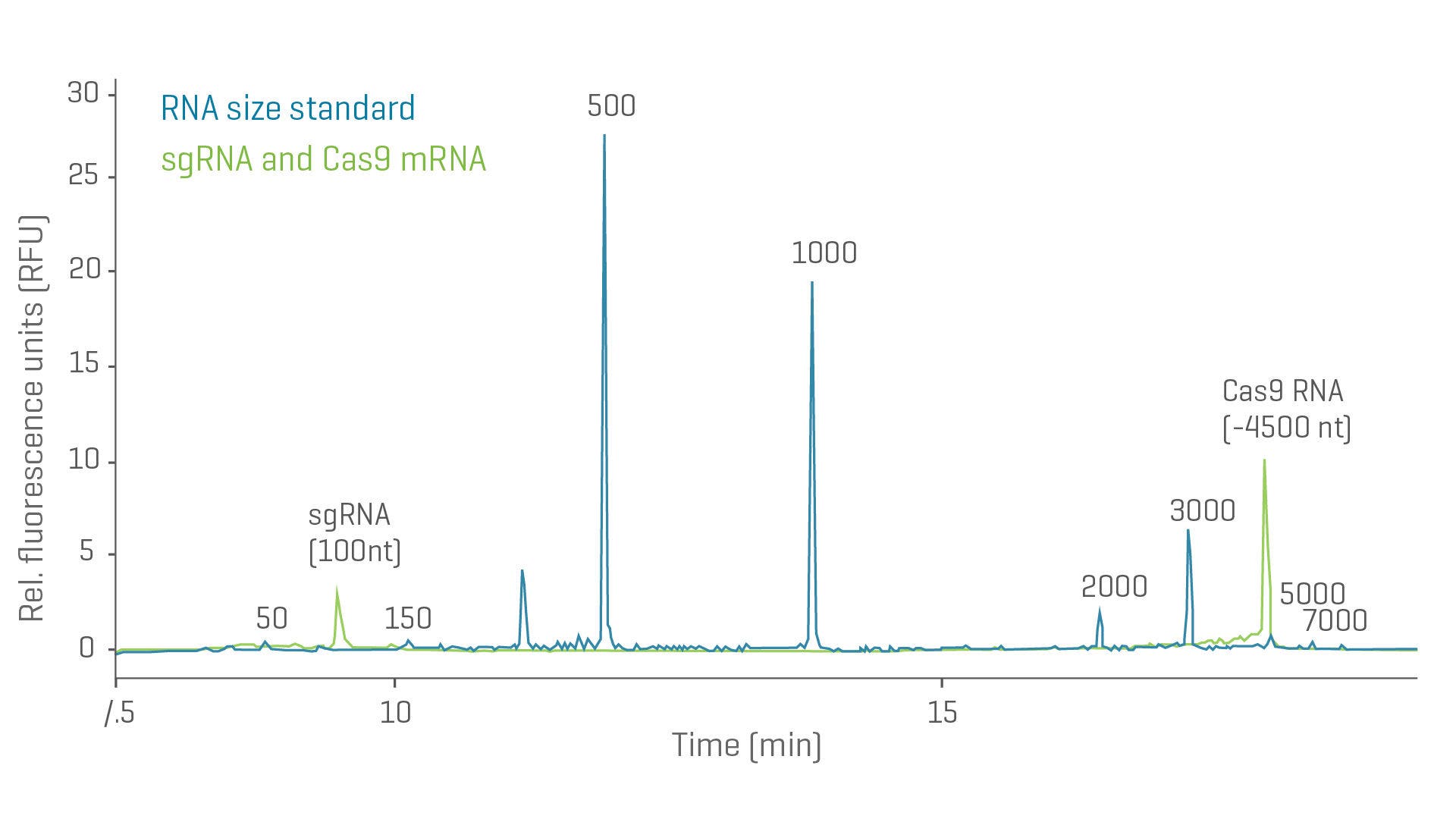
Purity and integrity of sgRNA and mRNA
- Simultaneously determine the purity and integrity of single-guide RNA (sgRNA) and mRNA for CRISPR gene editing applications
- Understand related nucleic acid impurities and determine sizes and quantities over a wide size range (50 to 9,000 nt)
- Cover your compliance needs through compatibility with common data management systems
Overlay of electropherograms from a mixture of sgRNA and Cas9 mRNA and an RNA ladder using the BioPhase 8800 system. The mRNA of ~4500 nt and the sgRNA of 100 nt can be assessed within the same analysis (green trace). The RNA ladder is shown in blue.
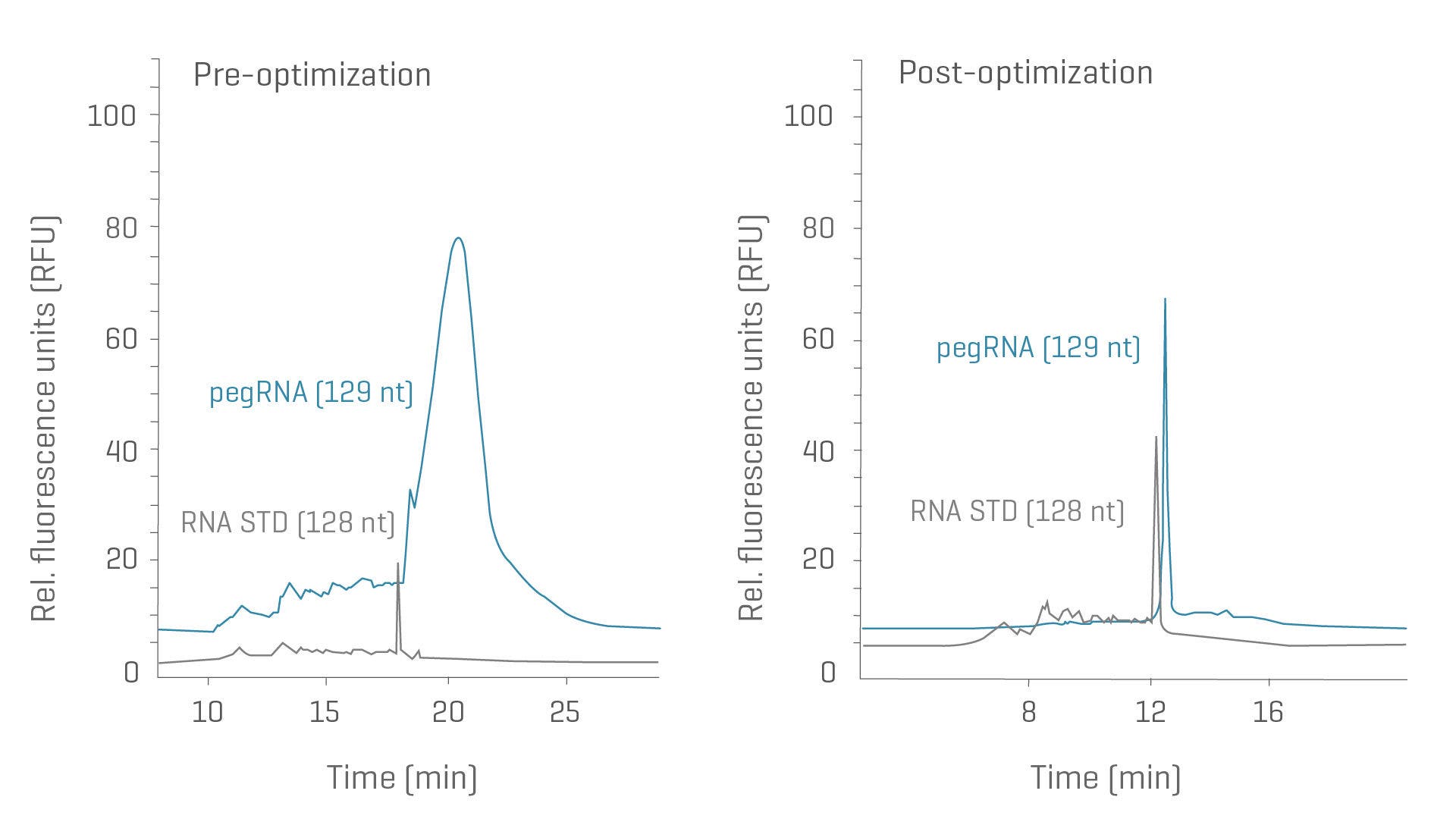
Prime editing guide RNA (pegRNA) purity
Achieve superior resolution and repeatability for purity assessments of intermediate products.
- Break through the boundaries of secondary structure with liquid-based temperature control
- Streamline data management and cover your compliance needs through compatibility with common data management systems
CGE analysis of pegRNA (blue trace) and noncomplementary RNA standard (STD, green trace) with similar length. Left: Before method optimization, the pegRNA (blue trace) shows extensive tailing compared to the noncomplementary STD (green trace). Right: After method optimization, the pegRNA sample shows a single sharp peak with comparable peak width as the non-complementary STD.
Purity of plasmid starting material with CGE
Assess linear DNA sizes and purity confidently with ultra-high resolution over a wide size range.
- Determine linearization efficiency with excellent separation for topological variants of pDNA
- Assess linear DNA sizes and purity confidently with ultra-high resolution over a wide size range
- Streamline data management through compatibility with data management systems
- Confidently transfer assays from development to QC
Linearized plasmid purity analysis and size estimation of a 7.9 kb plasmid sample. The electropherogram shows the sample prior to linearization, containing mainly the supercoiled isoform and after linearization.
Industry and regulatory-proven CE technology
“A sensitive, analytical method for quantitative estimation of product-related impurities such as nonglycosylated molecules, half antibodies, and fragments.” - USP referenced USP 39 NF 34 Chapter
Develop and lock down methods applicable to QC or regulated environments. The PA 800 Plus system easily transfers methods using robust, automated assays with proven portability—perfect for global multi-user, multi-instrument organizations
CE systems from SCIEX have earned a global reputation for consistently meeting or exceeding our customers’ quality expectations. Our commitment to excellence and continuous improvement is reflected in our comprehensive quality management system, which complies with the requirements of ISO 9001:2008.
Software
Meet data management needs with direct control. Confidently safeguard the success of your biologics with a system that enables regulatory compliance.
Reduce regulatory risk and maximize method transfer efficiency. Enable advanced integration of your PA 800 Plus system with the Empower Chromatography Data System using the PA 800 Plus driver for Empower software.
Boost your laboratory's productivity by seamlessly integrating it into a single software solution. Take control of your PA 800 Plus system with the Thermo Scientific™ Chromeleon™ driver and unleash next-level performance with powerful processing, customizable reporting, and robust compliant tools.
Experience unparalleled accuracy and efficiency in your capillary electrophoresis analysis with SCIEX 32 Karat software, the ultimate single-point controller for the PA 800 Plus system. It includes all current, tested methods for the PA 800 Plus system, covering IgG, CHO, cIEF, SDS MW, Fast Glycan, RNA, and performance applications.
Related biopharma applications
Reclaim time for innovation by identifying and advancing drug candidates more confidently with analytical strategies that support your team across modalities, from discovery to commercialization.
Break through barriers and extend the frontiers of mRNA, saRNA, and circRNA development with intuitive analytical solutions. Confidently innovate with high-quality, sensitive, and accurate data to assess integrity, purity, and CQAs.
Advance your projects with purpose-built solutions for the analysis of pDNA and dsDNA to support the development of your mRN), viral vectors, recombinant proteins, and other new modalities.
Optimize your processes with solutions that overcome the analytical challenges of viral vector-based drugs. Take control of full and empty ratios and protein and genome profiles. Break down the boundaries of protein and post-translational modification (PTM) characterization with dedicated high-resolution workflows offering unprecedented depth.
Set your own schedule for CRISPR/Cas9 gene editing with innovative, intuitive analytical solutions. Unleash the potential of your Cas9 mRNa, sgRNA, and Cas9 proteins by understanding quality, purity, and safety and assessing on/off target effects.
Set the pace with confidence. Cell line monitoring and analysis solutions verify the identity, purity, and stability of lead candidates with high throughput strategies that are ready for automation.
Fully understand drug candidate glycoprofiles with pipeline-specific solutions. Gather critical high-resolution information and site-specific data points with advanced glycan analysis for complex biologic development.
Pursue the end goal with confidence. Achieve the full picture and accelerate decisions with comprehensive characterization of biopharmaceutical charge variants.
Let's connect.
Stay up-to-date with biopharma conversations on LinkedIn