Set the pace with confidence. Cell line monitoring and analysis solutions verify the identity, purity and stability of lead candidates with high throughput strategies that are ready for automation.
Cell line monitoring and analysis

Explore the latest and trending topics
-
Technical note
Elevate CE-SDS analysis with native fluorescence detection.
The BioPhase 8800 system with native fluorescence detection (NFD) enhances CE-SDS assay performance by providing higher sensitivity, a stable baseline for easier peak integration, and excellent intra- and inter-capillary reproducibility in protein therapeutic analysis, ensuring robust, consistent quality attribute measurements.
-
Technical note
Improve baseline stability for cIEF analysis
Capillary isoelectric focusing combined with native fluorescence detection (cIEF-NFD) on the BioPhase 8800 system offers enhanced sensitivity, improved baseline stability, and excellent repeatability for protein charge heterogeneity analysis, demonstrating strong comparability with traditional UV detection while reducing ampholyte interference and facilitating automated data integration in biopharmaceutical applications.
Overview
Cell-line monitoring throughout drug development helps ensure quality in the biotherapeutic.
Gain the advantage of knowledge up front, with rapid, sensitive, and accurate cell line analysis strategies.
Capillary electrophoresis (CE) and mass spectrometry (MS) workflows address the need for speed, while providing accurate, quantitative information on cell culture media components and cell line stability.
Fast cell-line screening with CE
Workflow
Charge ahead to find candidates that make it to market.
Characterize critical properties, address throughput challenges and gain valuable quantitative data on cell-line samples with automation-friendly capillary electrophoresis (CE) workflows.
High-throughput purity, charge heterogeneity and N-glycan analysis CE workflows provide speed, reproducibility and resolution for product characterization during process and cell-line development.
- Enable quick, easy assessment of product quality
- Minimize sample preparation and method development
- Work toward automation with simple to integrate multi-channel formats
- Software templates optimized for peptide mapping and PQA selection
- Reduce regulatory risk with direct control through the Empower Chromatography Data System
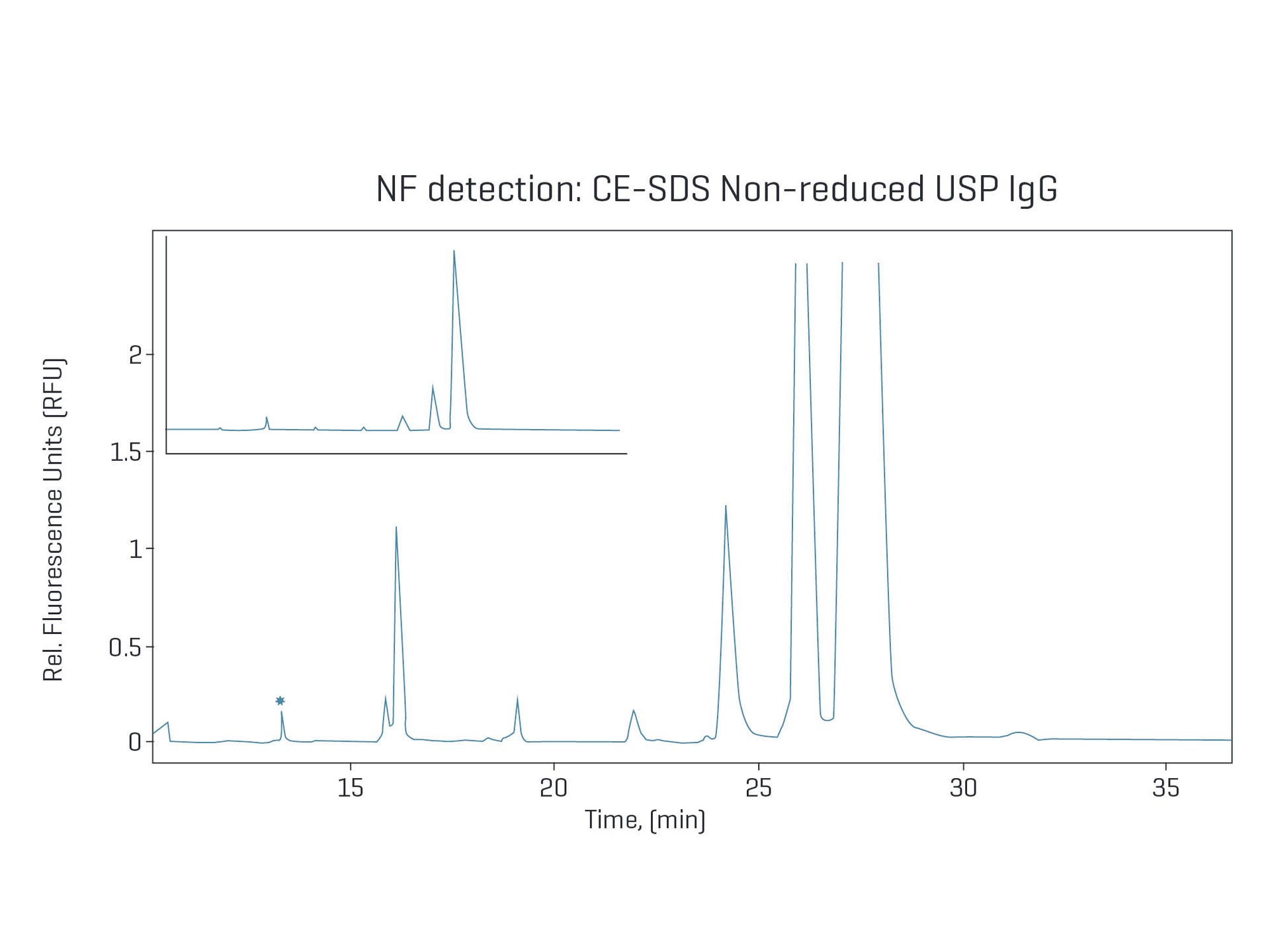
Non-reduced CE-SDS analysis of NIST
IgG with NF detection:
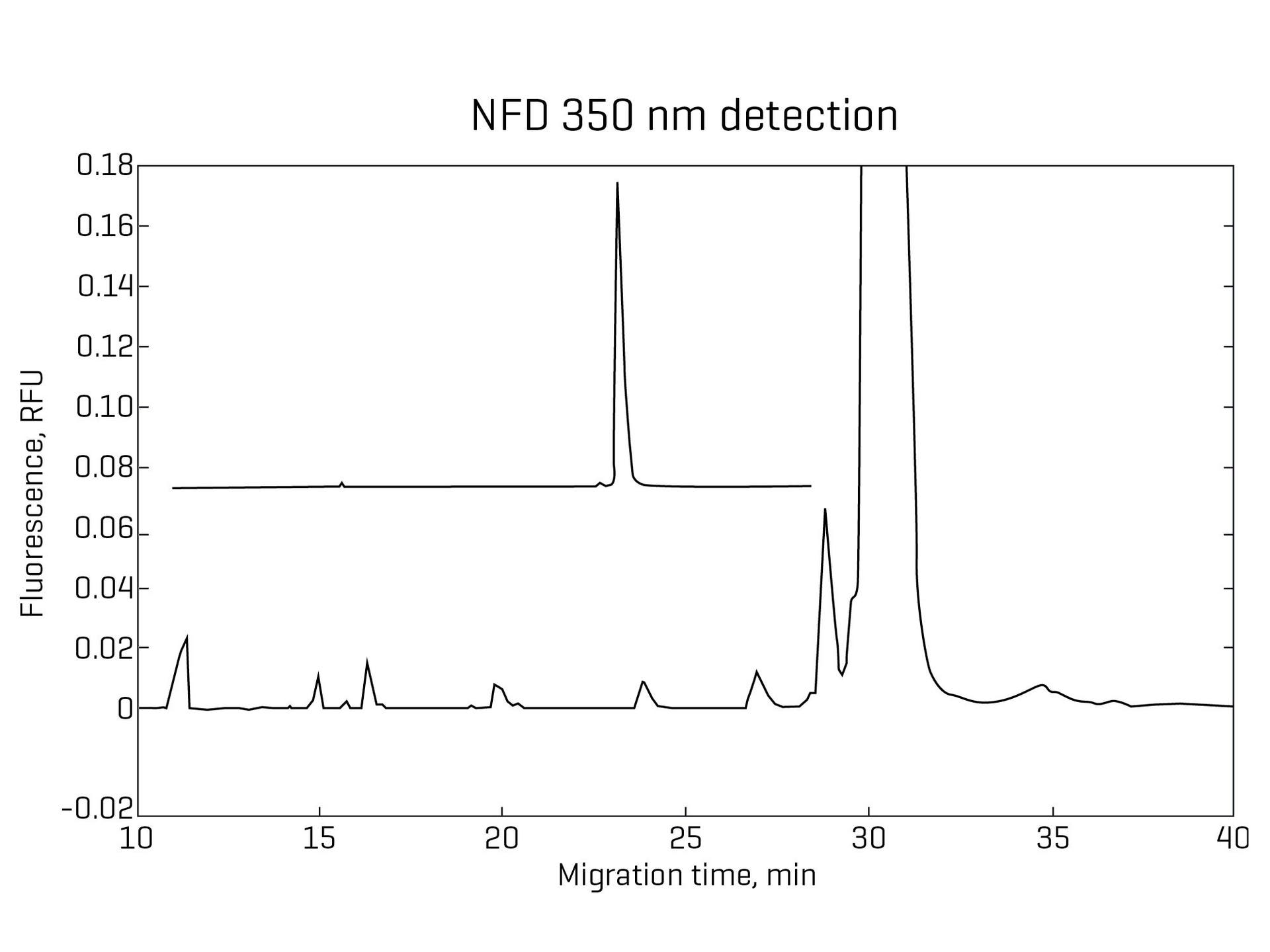
Improved signal-to-noise ratio with NF results in a stable baseline and up to 10X improvement in sensitivity, allowing for faster interpretation and integration of peaks.
Enable quantitation of low-level impurities
UV detection can struggle to identify low-level impurities due to interference from baseline noise. NF detection reduces the baseline noise and enables the quantitation of low-level impurities down to 0.01%.
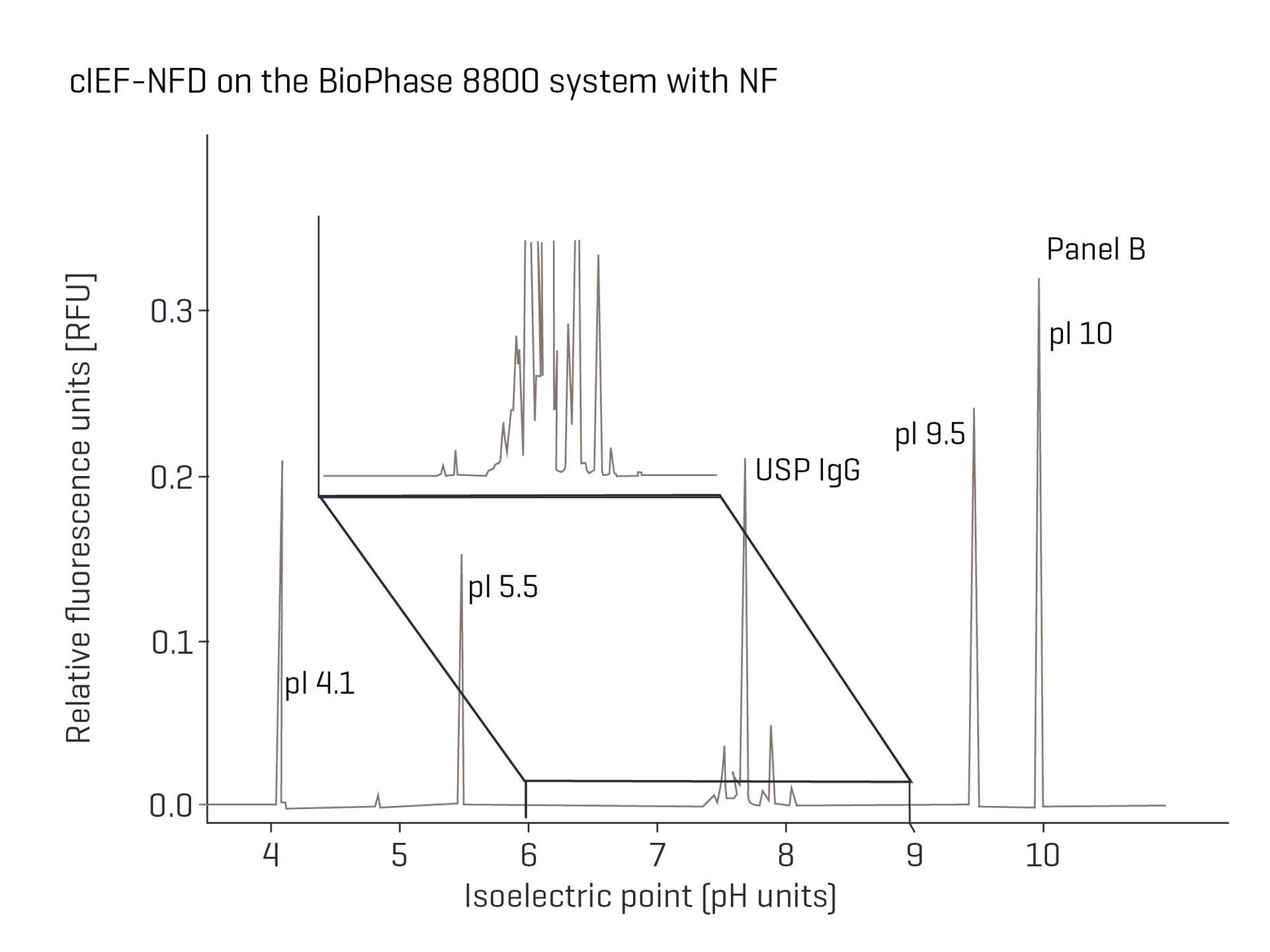
Charge heterogeneity analysis of NISTmAb on the BioPhase 8800 system with NF. Achieve equivalent data between UV and NFD for cIEF with a more stable baseline to enable better peak integration and identification of low abundant species.
Resources
-
Technical note
Elevate CE-SDS analysis with native fluorescence detection
The BioPhase 8800 system with NFD enhances CE-SDS assay performance by providing higher sensitivity, a stable baseline for easier peak integration, and excellent intra- and inter-capillary reproducibility in protein therapeutic analysis.
-
Technical note
Improve baseline stability for cIEF analysis
cIEF-NFD on the BioPhase 8800 system offers enhanced sensitivity, improved baseline stability, and excellent repeatability for protein charge heterogeneity analysis.
-
Technical note
Enable a streamlined, accurate and high-throughput glycan analysis assay
High-throughput glycan expression profiling with automation friendly sample preparation and labeling.
Fast cell-line screening with CE
Suited for:
- High-throughput purity analysis
- Rapid CE-SDS assay
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Effective quantification and determination of protein purity and size.
Fast cell-line screening with CE
Suited for:
- High-throughput charge heterogeneity analysis
- Rapid CE-SDS assays
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Experimental protein isoelectric focusing point (pI) and analysis of charge variants.
Fast cell-line screening with CE
Suited for:
- High throughput N-linked glycan analysis
- Rapid glycan expression profiling
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Release and label glycoprotein N-linked glycans for high-resolution capillary electrophoresis using laser-induced fluorescence (LIF) detection and an HR-NCHO separation matrix.
Monitoring of target CCM compounds with LC/MS
Workflow
Gain qualitative understanding of cell culture media (CCM) components.
Comprehensive information on CCM components sets the stage for success by helping to ensure optimal CCM conditions and the overall quality of the biotherapeutic.
LC-MS workflows designed to address the wide range of polarities and separation properties of CCM compounds provide a highly sensitive method to monitor target analytes in a single run.
- Improve detection and quantitation limits of low-level analytes
- Save sample and reduce matrix effect with less injection volume
- Identification and quantitation of over 110 compounds in a single run
Monitoring of target CCM compounds with LC/MS
Suited for:
- High-throughput monitoring of CCM compounds
- Identification of targeted CCM compounds
Reproducibility, reliability and carryover performance to match your quantitative workflows. Dependability you can count on, from injection to injection and batch after batch.
Setting a new standard for instrument resilience and robustness, the SCIEX 7500+ system gives you the confidence to face evolving analytical challenges. Tackle tight timelines with exceptional sensitivity and with the power to control your instrument downtime.
Unleash the analytical power of the next-generation software platform for data acquisition and processing.
All resources
-
Technical note
Take charge of your project timelines
Assess molecular liabilities more quickly with the BioPhase 8800 system, allowing rapid developability assessment to streamline the selection of candidates.
-
Technical note
Reproducible glycan analysis for clone selection
Enable reproducible separation of a 96-well plate in under 2 hours.
-
Technical note
Streamline CE-SDS workflows with kitted reagents and parallel processing
Significantly improve throughput and process 96-well plates in 12 hours with reproducible separation between instruments and kits.
-
Technical note
Eliminate labor-intensive benchwork and provide a higher throughput in N-linked glycan analysis
Streamlined N-linked glycan analysis that employs an automated sample preparation with a magnetic bead-mediated glycan workflow followed by capillary electrophoresis (CE).
-
Webinar
Facilitate clone selection with high-throughput glycan profiling
Discuss high-throughput strategies for glycan analysis to condense development timelines.
-
Technical note
Eliminate the bottleneck for drug purity analysis with high-throughput rapid CE-SDS purity analysis
Screen a large number of clones for lead clone selection with an automation friendly multi-capillary CE-SDS workflow.
-
Technical note
Accelerate cIEF method development from days to hours
Streamline evaluation of multiple factors and post-translational modifications in a single set of experiments with a multifactorial DoE approach.
-
Technical note
Enable a streamlined, accurate and high-throughput glycan analysis assay
High-throughput glycan expression profiling with automation friendly sample preparation and labeling.
-
Technical note
Identify over 110 compounds in under 20 mins
Improve detection of low-level target analytes with a single method.
-
Technical note
In-depth profiling of metabolites in CCM
Follow quantitative trends to demonstrate cell growth behavior at different stages of development.
-
Technical note
Gain insight into mAb structural changes with a CE subunit analysis workflow
Use CE-SDS and cIEF strategies to understand the structural changes that may occur in different domains of the antibody, and leverage this orthogonal approach to better assign charge variant identification.
-
Technical note
A Rapid, high-resolution lot release assay for co-formulated mAbs with CZE
Discover a novel workflow for high-speed lot release assay using capillary zone electrophoresis on the PA800 Plus system. This kit-based workflow provides quantitative results with simple sample preparation.
-
Technical note
High-Throughput, automated, and reproducible released glycan screening with the BioPhase 8800 system
Discover an automated workflow, powered by the Biomek i5 liquid handler and BioPhase 8800 system. This workflow ensures rapid, reproducible, and high-throughput N-linked glycan analysis—improving efficiency, minimizing user error, and delivering consistent results for large-scale therapeutic screening.
-
Technical note
Elevate CE-SDS analysis with native fluorescence detection.
The BioPhase 8800 system with native fluorescence detection (NFD) enhances CE-SDS assay performance by providing higher sensitivity, a stable baseline for easier peak integration, and excellent intra- and inter-capillary reproducibility in protein therapeutic analysis, ensuring robust, consistent quality attribute measurements.
-
Technical note
Improve baseline stability for cIEF analysis
Capillary isoelectric focusing combined with native fluorescence detection (cIEF-NFD) on the BioPhase 8800 system offers enhanced sensitivity, improved baseline stability, and excellent repeatability for protein charge heterogeneity analysis, demonstrating strong comparability with traditional UV detection while reducing ampholyte interference and facilitating automated data integration in biopharmaceutical applications.
-
Technical note
Discover benefits native fluorescence detection for cIEF analysis
A comparative study of capillary isoelectric focusing (cIEF) separation and sample repeatability on the BioPhase 8800 system using UV absorbance and native fluorescence detection (NFD), Both detection modes produce highly comparable and reproducible charge heterogeneity profiles for monoclonal antibodies, with NFD offering lower background noise and better sensitivity for low-abundance variants, while UV detection yields higher main peak signals but with greater variability.
Associated applications
Pursue the end goal with confidence. Achieve the full picture and accelerate decisions with comprehensive characterization of biopharmaceutical charge variants.
Fully understand drug candidate glycoprofiles with pipeline-specific solutions. Gather critical high-resolution information and site-specific data points with advanced glycan analysis for complex biologic development.