Obtain fast, quantitative data for purity and integrity analysis of therapeutic proteins.

CE-SDS purity analysis kits
Rapid determination of protein purity with capillary electrophoresis sodium dodecyl sulfate
CE-SDS is widely used throughout therapeutic drug development for lot release, stability testing, formulation-buffer screening, process development, cell line development and product characterization.
Maximize the way you work with validated kits and methods designed to optimize resolution and throughput.
Validated kits for CE-SDS workflows
SDS-MW analysis kit
The SDS-MW analysis kit is designed for use on the PA 800 Plus system and used for the separation and sizing of protein-SDS complexes.
Estimate molecular weight of an unknown protein
Quantify the amount of protein
Determine the purity of a protein product.
This kit uses a replaceable gel matrix formulated to provide an effective protein sieving range of approximately 10kDa to 225kDa.
Includes reagent volumes for analysis of 100 samples and components allowing for protein sizing.
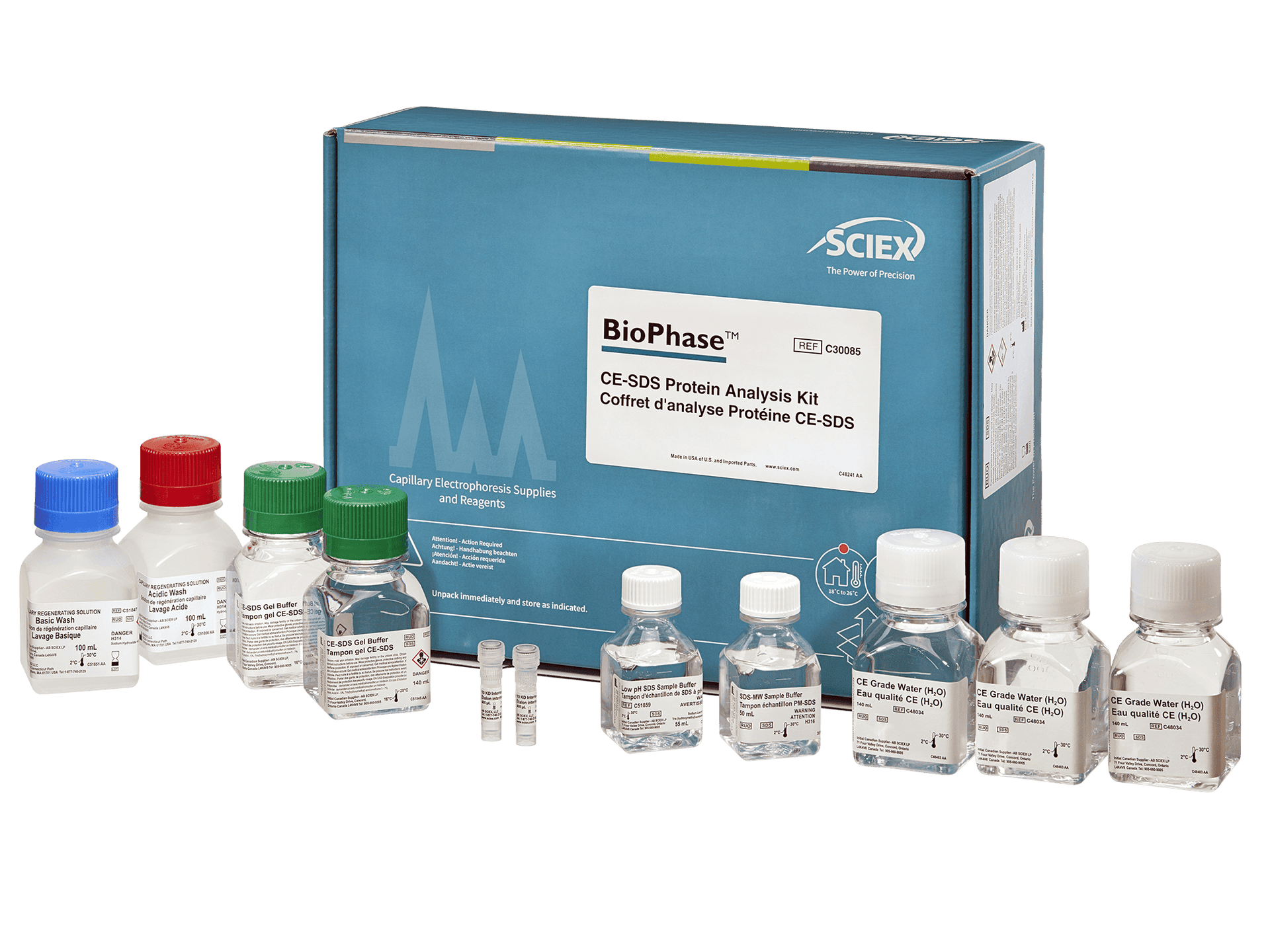
BioPhase CE-SDS protein analysis kit
As the drug development pipeline evolves, so do throughput needs. This kit is for use on the BioPhase 8800 system, designed to meet throughput demands by enabling 8 samples to be run in parallel, significantly increasing throughput and eliminating analytical bottlenecks.
Flexible workflows tailored for high-throughput CE-SDS analysis, for the BioPhase 8800 system optimize time to answer while ensuring excellent intermediate precision, accuracy, and separation efficiency.
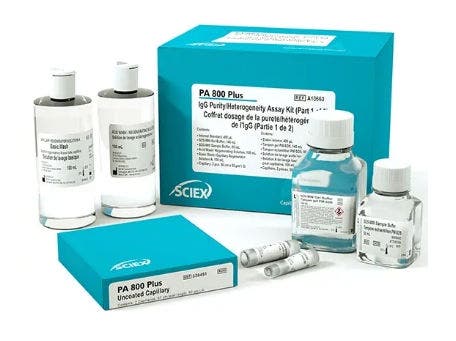
IgG purity and heterogeneity kit
This kit is designed for use on the PA 800 Plus and is used to assess the purity and heterogeneity of IgG molecules in both a reduced and non-reduced state, and will detect impurities as low as 0.1%.
This kit provides reagent volumes for analysis of 100 samples.
Compatible products
Characterize therapeutic molecules with confidence with the kit-based system.
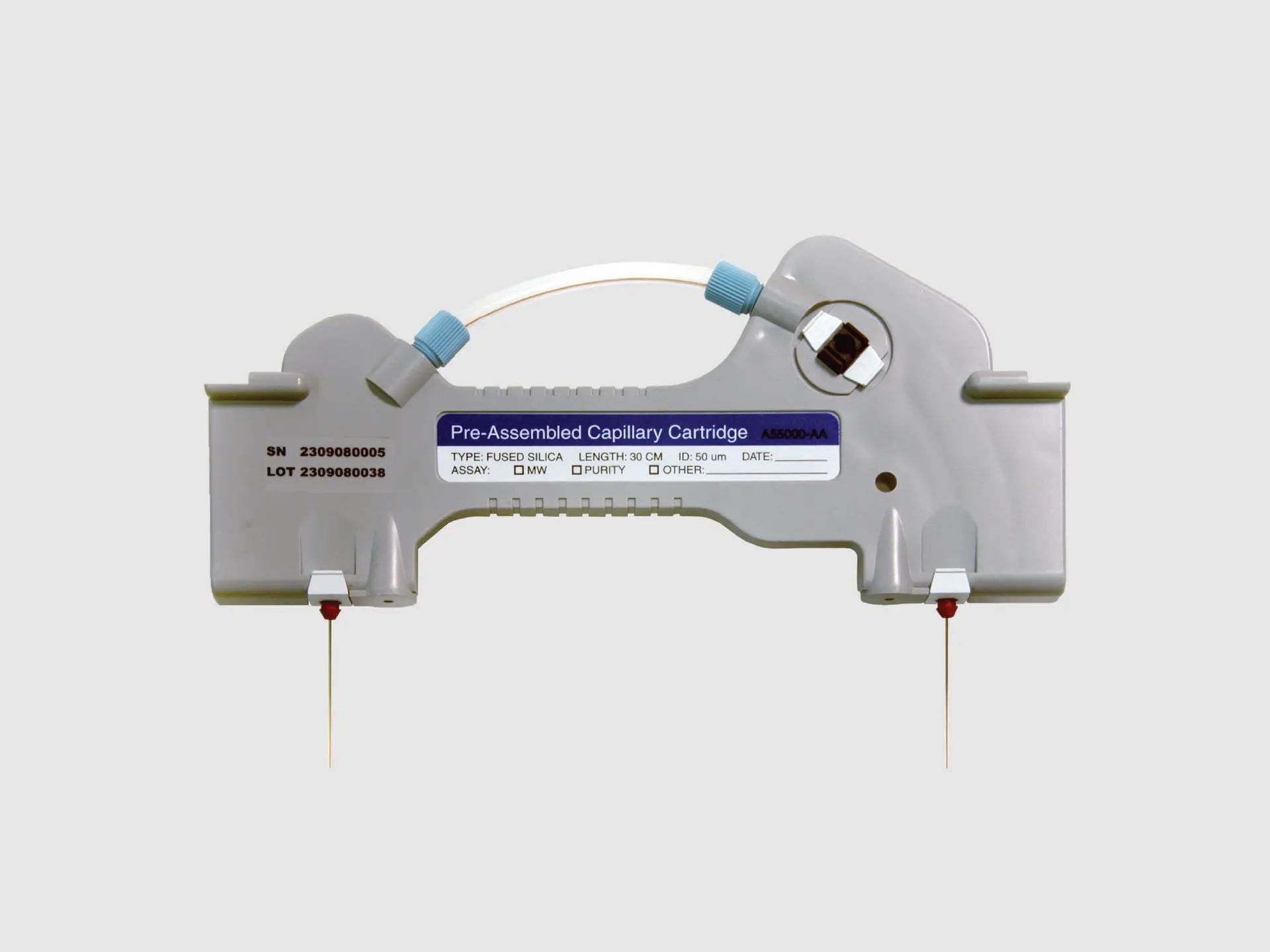
Pre-assembled bare-fused silica capillary cartridge compatible with the RNA 9000 Purity & Integrity kit and the PA 800 Plus system
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Pre-assembled bare-fused silica 8-capillary cartridge for the BioPhase 8800 system - 8 x 30 cm. (P/N 5080121)
Resources
-
Technical note
Intermediate precision study of capillary electrophoresissodium dodecyl sulfate (CE-SDS) assay on the BioPhase 8800 system
-
Technical note
Lightning capillary electrophoresis sodium dodecyl sulfate (CE-SDS) workflow for high-throughput analysis of biotherapeutics
-
Technical note
Unleashing the Power of Speed: Accelerating time to answer with CE-SDS and Laser-Induced Fluorescence detection in under 12 Minutes
-
Technical note
Multi-capillary gel electrophoresis to increase throughput for the purity analysis of biotherapeutic proteins
-
Technical note
Efficient transfer of the high‐speed CE‐SDS method from the PA 800 Plus system to the BioPhase 8800 system
-
Technical note
Automation of CE-SDS sample preparation for PA 800 series IgG purity/ heterogeneity assays
-
Technical note
Assay of IgG purity and heterogeneity using high-resolution sodium dodecyl sulfate capillary gel eletrophoresis
-
Technical note
High precision lentivirus titer determination and protein profiling
-
Technical note
Elevate CE-SDS analysis with native fluorescence detection.
The BioPhase 8800 system with native fluorescence detection (NFD) enhances CE-SDS assay performance by providing higher sensitivity, a stable baseline for easier peak integration, and excellent intra- and inter-capillary reproducibility in protein therapeutic analysis, ensuring robust, consistent quality attribute measurements.
-
Technical note
Improve baseline stability for cIEF analysis
Capillary isoelectric focusing combined with native fluorescence detection (cIEF-NFD) on the BioPhase 8800 system offers enhanced sensitivity, improved baseline stability, and excellent repeatability for protein charge heterogeneity analysis, demonstrating strong comparability with traditional UV detection while reducing ampholyte interference and facilitating automated data integration in biopharmaceutical applications.