Abstract
There are various challenges during lentivirus production including virus stability, toxicity of the virus to the host cell lines, and limited ability to properly characterize the active virus product. As a result, monitoring product quality is critical. Titer is one of the critical quality attributes (CQA) for lentivirus production from lab scale to GMP manufacturing. This technical note describes a method that offers a more accurate titer for lentivirus than ELISA and can also be utilized for profile-based purity assessment. This easy-to-use SDS-CGE method is aso amenable to automated analysis of the Lentiviral proteome with high resolution, excellent quantitation capability and great reproducibility. An easily applicable two step sample preparation and fluorescent labeling protocol is also described.
Introduction
In this technical note, we introduce a high-performance sodium dodecyl sulfate-capillary gel electrophoresis based assay, using the SCIEX PA 800 Plus pharmaceutical analysis system in conjunction with the SDS-MW protein analysis kit for highly precise lentivirus titer determination and profiling. This instrument with the associated kit provides automated separation of proteins in the size range of 10 kD to 225 kD with high resolution, excellent quantification capability and great reproducibility. Along with the separation method, we also describe an easy sample preparation protocol including the denaturing and labeling steps for quantitative determination of the lentivirus P24 protein using a pyrylium dye. This dye readily reacts with the primary amines of the polypeptide backbone of proteins and also features very low (<1%) fluorescence in its unconjugated format, thus decreasing baseline noise and increasing detection limit.
Lentivirus, a member of the retrovirus family 1, is capable of integrating large DNA molecules into host cells, thus representing one of the most efficient gene delivery vehicles for transduction 2. For this particular application, lentivirus possesses several advantages, including its ability to infect both non-dividing and dividing cells. For clinical therapeutic applications, the stable and long-term transgene expression of these retroviruses represent a great advantage 3. The proteome of the lentivirus is composed of five important structural and several non-structural proteins. The vital structural proteins are as follows: gp120 surface envelope protein (120 kDa), gp41 transmembrane envelope protein (41 kDa), p24 capsid protein (24 kDa), p17 matrix protein (17 kDa) and the p7/P9 nucleocapsid protein (7-11 kDa). The two envelope proteins of gp120 and gp41 are highly glycosylated. This post translational modification (PTM) apparently conceals important antigenic sites, thus helps the virus to avoid recognition by the immune system of the host. The p24 protein is the building block of the lentivirus capsid and approximately 2000 of them assemble the full capsid.
The various challenges during lentivirus production include virus stability, toxicity of the virus to the host cell lines, and limited ability to properly characterize the active virus product. As a result, monitoring product quality is critical. Titer is one of the critical quality attributes (CQA) for lentivirus production from lab scale to GMP manufacturing.
Key features
- Sensitive titer determination based on precise quantification of the separated p24 protein peak
- Easy batch-to-batch reproducibility and purity assessment based on the resulting proteome profile
- Efficient two-step sample preparation protocol including denaturation and covalent fluorophore labeling of the lentivirus proteome with no sample cleanup requirement
- Excellent repeatability with 0.20% RSD on migration time, and less than 0.91% on Corrected Peak Area%
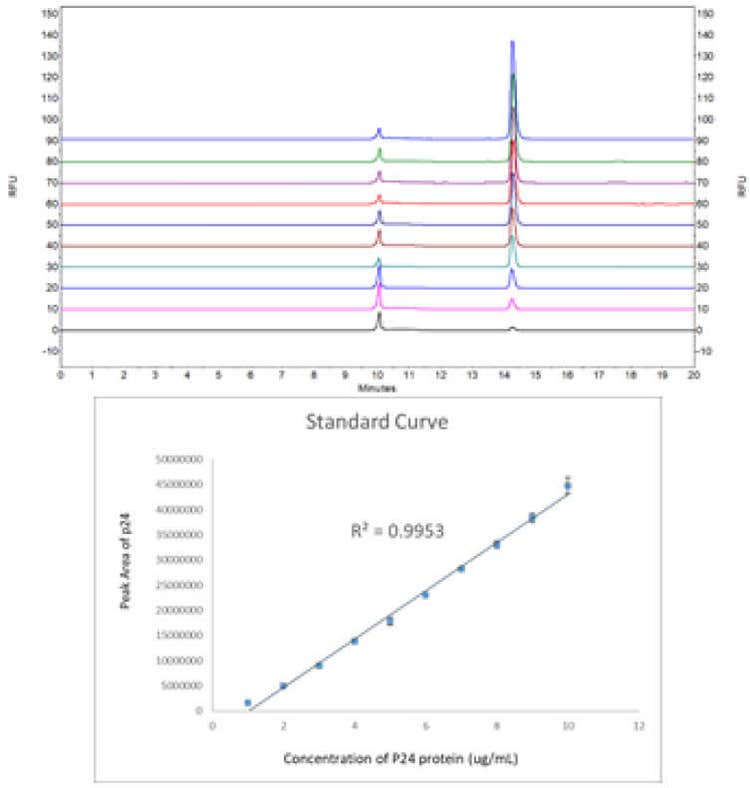
- Good titer determination detection linearity of (r2 = 0.9953) in the concentration range of 1-10 μg/mL
- The method offers the LOQ of 8 ng/mL for p24
Measuring the functional titer of a recombinant lentivirus is not only dependent on how efficiently the transfer vector is packaged but also the transduction efficiency of the cell line used 4. Quantification (titer determination) of a lentivirus to ensure efficient expression is usually based on the quantification of the p24 protein. ELISA assay is the traditional method for lentiviral titer determination, aiming for this particular protein. However, this method measures virus-associated p24 along with possible false positives with similar epitopes, resulting in overestimation of the lentivirus titer. This calls for an orthogonal method to be used in combination with the regularly used screening ELISA test. The orthogonal method can be a separation-based technique, in which case it can be used to recognize false positives 5, to provide more accurate titer, and concomitantly utilized for profile-based purity assessment.
To address these issues, we describe an easy-to-use SDS-CGE method for high precision lentivirus titer determination along with its protein profiling-based purity assessment. The approach offers automated analysis of the lentiviral proteome with high resolution, excellent quantification capability and great reproducibility. An easily applicable two-step sample preparation and fluorescent labeling protocol is also described.
Materials and methods
Materials: Pre-made lentivirus (LV-CAG-GFP, PN SL100270) was from SignaGen Laboratories (Rockville, MD) and the HIV1 p24 protein (PN ab127888) was purchased from Abcam (Cambridge, UK). The Chromeo P503 dye (P/N 15106) was from Active Motif (Carlsbad, CA). Phosphate Buffered Saline (PBS) 10x bioreagent suitable for cell culture (P/N P5493-1L) was from Sigma-Aldrich (St. Louis, MO). The SDS-MW Analysis Kit (P/N 390953) was from SCIEX (Framingham, MA), including the SDS-MW gel buffer, the acidic and basic wash solutions (0.1 N HCl and 0.1 N NaOH) and the SDS-MW sample buffer of 100 mM Tris-HCl (pH 9.0) with 1% SDS.
Sample preparation: Preparation of the Chromeo P503 working solution: 1 mg of Chromeo P503 dye (lyophilized powder) was reconstituted in 1 mL of methanol. After reconstitution, the dye label can be stored at 2-8°C for six months according to the manufacturer’s instructions. Sample preparation: 5 μL of lentivirus sample solution (1.5 x 109 TU/mL) was mixed with 5 μL of sample preparation solution provided in the SDS-MW Analysis Kit and incubated at 70°C for 10 minutes followed by mixing with 0.5 μL of Chromeo P503 dye working solution and incubated again for another 10 minutes at 70°C. After cooling down to room temperature, 39.5 μL of DI water was added to the reaction mixture and the diluted sample was transferred to the sample vial for SDS-CGE-LIF analysis.
Capillary electrophoresis: A PA 800 Plus pharmaceutical analysis CE system (SCIEX) equipped with a laser-induced fluorescence (LIF) detector with a 488 nm solid state laser and a 600 nm emission filter was used for all separations. The pre-assembled EZ-CE capillary cartridge (SCIEX, P/N A55625) had a bare fused-silica capillary with 50 μm I.D. and 30 cm total length (20 cm effective length). The SCIEX universal vials (P/N A62251), universal vial caps (P/N A62250) and PCR vials (P/N 144709) were used for sample loading with the following injection parameters: water stacking at 20.0 psi for 0.40 min and injection at 5kV for 60 s. The detailed information of condition, separation and shutdown methods are shown in Figure 2-6. Data acquisition and analysis were performed using 32 Karat software version 10.
Results and discussion
Lentivirus is a spherical enveloped virus with an average diameter of 80–100 nm and approximately 8 nm long spikes at the surface (Figure 6). Inside the envelope is an isometric capsid, assembled from p24 proteins to hold a rod-shaped nucleic acid payload possessing gag, pol and env genes, coding for all viral proteins 6. Quantification of this p24 protein was used in this study for high precision lentivirus titer determination. Albeit, the sensitivity and specificity of immunoassay based methods have been increasing in the past decades, cross reactivity based overestimation of the analyte molecules still represent a problem 7. Introducing an orthogonal separation-based titer determination method alleviates many of the limitations of the ELISA assays 8.
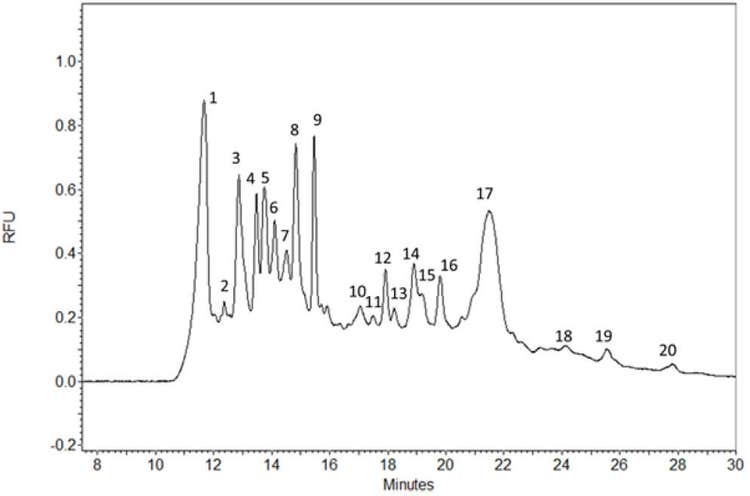
The titer determination workflow started with an efficient two-step sample preparation protocol, including the denaturation and covalent fluorophore labeling steps as described in the Experimental section. The resulting pyrylium dye tagged lentivirus proteome was then subject to quantitative sodium dodecyl sulfate-capillary gel electrophoresis analysis. The resulting electropherogram is shown in Figure 1, featuring 18 peaks. Since most of the lentivirus proteins possess various post-translational modifications (PTMs), such as glycosylation, standard curve based molecular mass assessment using the sizing ladder was not considered to be adequate, thus was not pursued in this study.

Based on the apparently good detection response during the spiked reproducibility study, the limit of quantification (LOQ) values were determined to be 8 ng/mL for p24 by injecting a series of 2x dilutions of standalone p24 standard into the SDS-MW gel-filled capillary column.
For 6 consecutive injections of the lentivirus sample solution, the RSD% of MT and corrected peak area of P24 peak were calculated to be 0.20% and 0.91%, respectively, as shown in Table 1.

Conclusions
- In this technical note we introduced a rapid, two-step sample preparation protocol, along with the optimized separation parameters for the fluorophore labeled lentivirus proteome for capillary electrophoresis analysis with laser induced fluorescent detection
- Quantification of the p24 protein was used for precise lentiviral titer determination
- Comparing the ELISA and SDS-CGE data revealed a probably incorrect number for the former, possibly due to some cross-reactivity during the immunoassay-based method
- The inherent titer overestimation of the ELISA kit can be readily alleviated with the use of the SDS-CGE method described here
- The method introduced here features excellent migration time and corrected peak area repeatability of 0.20% RSD and 0.91% RSD, respectively, with excellent titer determination detection linearity of r2 =0.9953 in the concentration range of 1 μg/mL-10 μg/mL
- The LOQ of the method was 8 ng/mL for the p24 capsid protein.
References
- Telesnitsky, A., Retroviruses: Molecular Biology, Genomics and Pathogenesis. Future Virol, 2010. 5(5): p. 539-543.
- Cockrell, A.S. and T. Kafri, Gene delivery by lentivirus vectors. Mol Biotechnol, 2007. 36(3): p. 184-204.
- Anguela, X.M. and K.A. High, Entering the Modern Era of Gene Therapy. Annu Rev Med, 2019. 70: p. 273-288.
- Geraerts, M., et al., Comparison of lentiviral vector titration methods. BMC Biotechnol, 2006. 6: p. 34.
- Guzman, N.A. and D.E. Guzman, A Two-Dimensional Affinity Capture and Separation Mini-Platform for the Isolation, Enrichment, and Quantification of Biomarkers and Its Potential Use for Liquid Biopsy. Biomedicines, 2020. 8(8).
- Durand, S. and A. Cimarelli, The inside out of lentiviral vectors. Viruses, 2011. 3(2): p. 132-59.
- Tate, J. and G. Ward, Interferences in immunoassay. Clin Biochem Rev, 2004. 25(2): p. 105-20.
- Terato, K., et al., Preventing intense false positive and negative reactions attributed to the principle of ELISA to re-investigate antibody studies in autoimmune diseases. J Immunol Methods, 2014. 407: p. 15-25.




