Abstract
This technical note discusses how the IgG Purity/Heterogeneity method using CE-SDS on a PA 800 Plus Pharmaceutical Analysis System can be easily adapted to accommodate 96-well plate preparations. The use of the 96-well plate allows for an increase in instrument sample throughput by allowing up to 100 injections per run sequence. This also allows for a reduction in analyst sample preparation time as it is multi-channel pipette compatible, which can help reduce pipetting errors and inter-sample variability.

Introduction
With higher sample throughput becoming an ever-increasing need in the biopharmaceutical industry, a modification to the tray layout (figure 1) of the IgG Purity/Heterogeneity assay or SDS molecular weight (SDS-MW) assay1,2 is all it takes to accommodate a full 96-well plate and increase the throughput of analysis without compromising assay sensitivity or reproducibility. This increase in throughput is ideal for those occasions when you have large sample sets or are in an instrument limited environment and need to push instrument capacity to its limit.
96-well plate preparations also allow for the added benefit of an easier, faster sample preparation using multi-channel pipettes and is automation friendly.3 The modification to the tray layout also allows for a decrease in cost per sample analyzed due to fewer reagents and vials being used in the analysis.
This technical note describes the sample preparation and instrument parameters necessary to implement 96-well plate analysis of the IgG purity/heterogeneity and SDS-MW assays.
Key features of 96-well plate format
- Decrease sample preparation time by over 50% compared to single pipette preparation
- Increase instrument throughput by 4x (96 vs 24)
- Lower consumable consumption per analysis
- Lower analyst preparation time
- Maintain high assay reproducibility
- Automation compatible preparation
Materials and Methods
Instrumentation: All experiments were performed on the PA 800 Plus Pharmaceutical Analysis System (SCIEX). Bare, fused-silica capillaries of 50 μm ID × 10 cm to detection were used for the separation.
Reagents: SDS-gel buffer, SDS sample buffer and the 10 kD protein internal standard were all manufactured at SCIEX (Carlsbad, CA). 2-mercaptoethanol and iodoacetamide were obtained from Sigma (St. Louis, MO). The gel buffer comprised a proprietary polymer buffer formulation (at pH 8.0) with 0.2% SDS. The sample buffer for reduced analysis composed of 100 mM Tris-HCl, pH 9.0, with 1% SDS. The sample buffer for non-reduced analysis composed of 70mM citrate-phosphate, pH 6.7, with 1% SDS. The acidic wash solution was 0.1 N HCl. The basic wash solution was 0.1 N NaOH.
The E-Z CE Cartridge (part # A55625) and 96-well microplate (part # 609844) were manufactured by SCIEX (Carlsbad, CA). The 96-well plate foil (part # 1814045) seal was manufactured by Bio-Rad (Hercules, CA). The 96-well plate pre-scored adhesive film (part # EZP-100) was manufactured by Excel Scientific (Victorville, CA).
Preparation of SDS-Protein Complex: For the reduced sample preparations, the protein sample (10 mg/mL concentration) was diluted with SDS-MW sample buffer to give a final concentration of 1 mg/mL. The IgG-SDS complex was reduced by adding 5% neat 2-mercaptoethanol (v/v), mixed, and heated on a heat block measuring 70°C for 10 minutes.
For the non-reduced sample preparations, the protein sample (10 mg/mL concentration) was alkylated with iodoacetamide by adding 5% of a 250 mM iodoacetamide (v/v) solution into the sample, followed by the addition of the non-reduced sample buffer. The solution was mixed and heated on a heat block measuring 70°C for 5 minutes.
Separation and Analysis: An optimized separation method and sequence were created for high speed (10cm separation) batch analysis of 96 samples at a time. For each separation cycle, the capillary was first preconditioned with 0.1 N NaOH, 0.1 N HCl, deionized water, and SDS gel buffer. Samples were electrokinetically introduced by applying voltage at 5 kV for 20 seconds. Electrophoresis was performed at 15kV with a one minute ramp time in a capillary thermostatted to 25°C using recirculating liquid coolant. The system was programmed to automatically replenish all reagents through a new method after every twenty five cycles.
Additional separation methods can be configured for high resolution (20cm separation) analysis and for analyses using a vacuum injection.
Results and Discussion
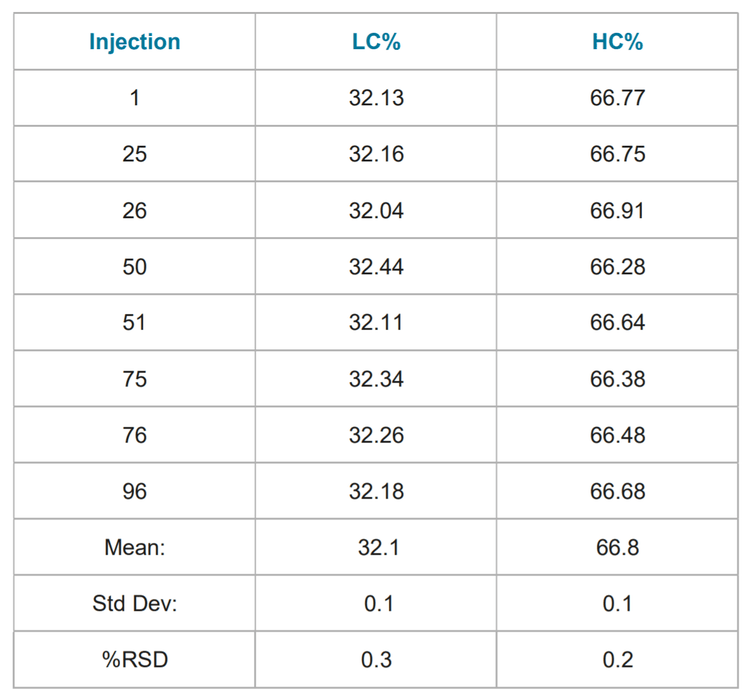
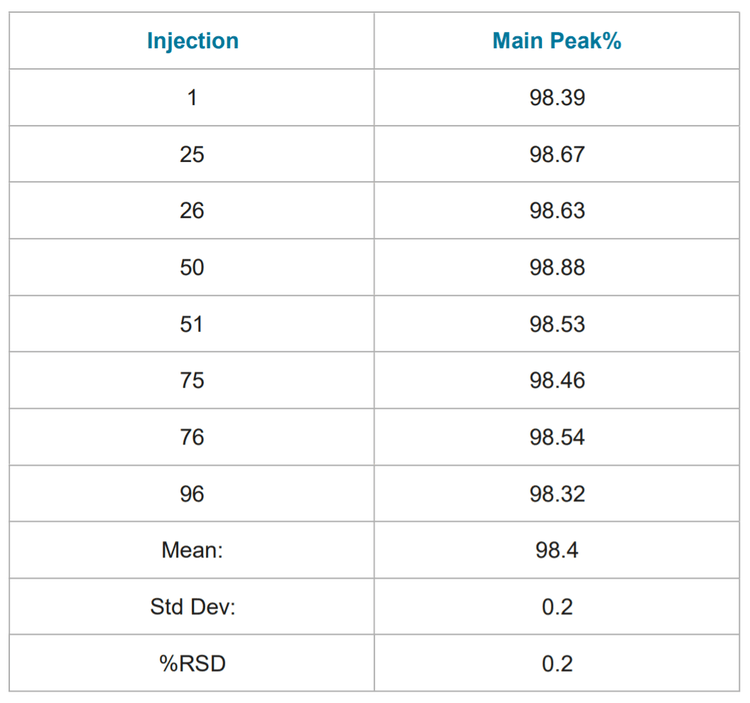
Assay Precision: Table 1 summarizes the results of ninety six consecutive analyses of the reduced NIST mAb. Table 2 summarizes the results of ninety six consecutive analyses of the non-reduced NIST mAb. The relative standard deviation (% RSD) of both the non-reduced and reduced purities were < 1%. The range of non-reduced purity values were within 0.5% of the reported value on the provided CoA for Reference Material 8671 (98.47%).
Technical Notes:
- The sample storage temperature was set to 10°C to reduce sample evaporation over the extended length of the sequence.
- Sample separation time should be increased for the high resolution method from 35 to 40 minutes due to inherent migration time shifting and to properly capture the aggregate peak.
- The 96-well plate must be covered to prevent sample evaporation.
- • Sample heating time may need to be evaluated if switching from a water bath to a heat block.
- Addition of mineral oil (Sciex part # 608114) to the SDSMW Gel buffer vials can be used to limit the inherent migration time shifting seen due to evaporation.
- The results shown are from the analysis of the NIST mAb. Preparation and instrument parameters may need to be independently optimized to achieve optimal results for each molecule.
Summary
The IgG Purity/Heterogeneity method can be easily adapted to accommodate 96-well plate preparations. The use of the 96-well plate allows for an increase in instrument sample throughput by allowing up to 100 injections per run sequence. This also allows for a reduction in analyst sample preparation time as it is multi-channel pipette compatible, which can help reduce pipetting errors and inter-sample variability.
The reproducibility of the separation method was demonstrated by performing 96 separations of NIST mAb in both reduced and non-reduced conditions. In these separations, the separation gel has been shown to be able to separate up to 25 samples before switching buffer rows, resulting in a decrease in cost per sample analyzed due to fewer consumables being used in the analysis.
References
- PA 800 Plus Pharmaceutical Analysis System-IgG Purity/Heterogeneity Assay Application Guide. SCIEX part number A51967AD, January 2014
- PA 800 Plus Pharmaceutical Analysis System-SDSMWAnalysis Assay Application Guide. SCIEX part number A51970AD, January 2014
- Automation of CE-SDS Sample Preparation for PA 800 Series IgG Purity/Heterogeneity Assays Using a Biomek 4000 Automation Workstation. SCIEX document number RUO-MKT-02-6959-A, November 2017
