Abstract
This technical note highlights the reproducibility of a streamlined icIEF-UV/MS workflow for charge variant separation, UV quantitation, and peak identification of monoclonal antibodies (mAbs) by Intabio ZT system coupled to ZenoTOF 7600 system. The icIEF-UV/MS measurements of NISTmAb were performed across 45 injections with 3 cartridges using the Intabio ZT system, resulting in high inter- and intra-cartridge reproducibility (%CVs <5%).
Recombinant mAbs are widely used in biotherapeutic applications due to their high specificity and efficacy. One challenge associated with the characterization of antibody-based biotherapeutics is sample heterogeneity caused by physiochemical transformations and post-translational modifications (PTMs) that might occur during mAb manufacturing.1 Monitoring and characterizing the heterogeneity of mAbs is important to assess critical quality attributes (CQA) of biotherapeutics to ensure drug safety and efficacy.
The Intabio ZT system offers direct chip-based integration of icIEF-UV with ZenoTOF 7600 system that can deliver rapid and reproducible separation and characterization of intact mAb charge variants, and assessment of proteoforms. 2,3 The Intabio ZT system coupled with ZenoTOF 7600 system offers a streamlined workflow for reproducible separation, identification and relative quantitation of mAb charge variants in a high-throughput manner. 3,4
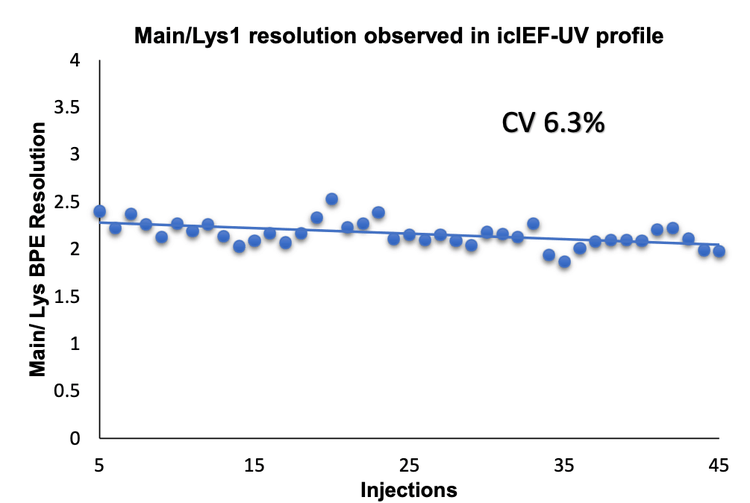
In this technical note, the icIEF-UV/MS workflow was employed to provide reproducible separation of the main, acidic and basic variants of NISTmAb across 45 injections using 3 different cartridges (Figure 1).
Key features of the icIEF-UV/MS workflow
- Reproducible separation, identification and relative quantitation of mAb charge variants with a seamless, microfluidic chip-based integrated icIEF-UV/MS workflow offered by the Intabio ZT system
- The 30-min sample analysis is significantly faster than conventional cIEF and IEX workflows requiring fractionation for the following identification.
- icIEF separation and UV relative quantitation correlate well with standard icIEF techniques
- Streamlined, intuitive data analysis software for rapid reporting and results sharing
Methods
Equipment: Intabio ZT system (SCIEX) and Intabio ZT cartridge (SCIEX, P/N 5088248) were used for the separation of NISTmAb and its charge variants. MS detection was performed on the ZenoTOF 7600 system (SCIEX, P/N 5080337) equipped with OptiFlow interface components (SCIEX, P/N 5084645).
Chemicals and reagents: The Intabio system – Electrolyte and Mobilizer kit (P/N 5088205) was used for anolyte, catholyte and mobilizer. Anolyte and mobilizer were used undiluted. The stock catholyte solution was 1% and diluted to 0.25% for use in the reagent drawer. The stock anolyte is 1% formic acid and catholyte is 1% diethylamine. The mobilizer is composed of 25% acetic acid25% acetonitrile and 50% water.
A 500mM cathodic spacer solution containing free base L-arginine (Arg) (purity ≥ 98.5%, Sigma-Aldrich, P/N A8094-25G) was prepared by dissolving 0.870 mg of Arg powder into 10 mL of Milli-Q water. The electrolytes and cathodic spacer solutions were stored at room temperature. pI markers (CanPeptide) were individually dissolved in Milli-Q water at 5 mg/mL.
Prior to icIEF-UV/MS analysis, NISTmAb was desalted with a Zeba Spin Desalting Columns, 7K MWCO, 0.5 mL (Thermo Fisher Scientific, P/N 89882).
Samples containing 400 µg/mL NISTmAb, 10mM Arg, 1 % Pharmalyte 3 to 10 (Cytiva P/N 17045601), 2.5% Pharmalyte 8 to 10.5 (Cytiva, P/N 17045501) and 6.0 µg/mL peptide pI markers were vortexed and then degassed by centrifugation at 3900 cf.
icIEF-UV/MS analysis: For the reproducibility test, 400 ug/mL NISTmAb solution was mixed with carrier ampholytes, and internal pI markers. The sample was separately analyzed with 3 different Intabio ZT cartridges.
The icIEF separation was achieved using the parameters shown in Table 1. UV absorbance measurements were collected at 1 Hz during the focusing and mobilization steps. The samples were introduced into the ZenoTOF 7600 system with a metered 2 µL/min flow of chemical mobilizer. The TOF MS data were acquired using the parameters shown in Table 2.
Results and discussion
Intra- and inter-cartridge relative abundance reproducibility
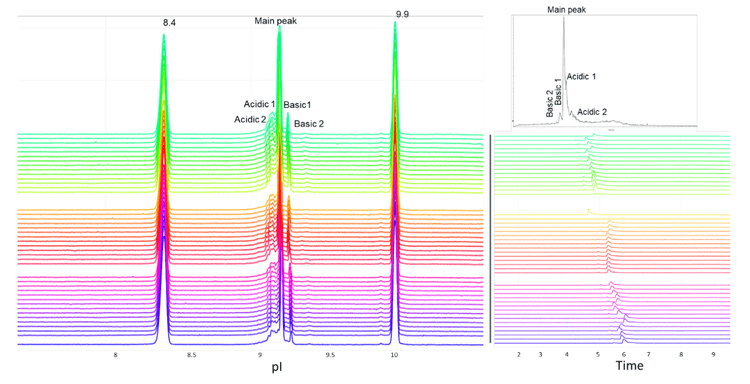
The combination of icIEF separation with UV quantitation and MS identification provides a high-resolution separation and comprehensive characterization of intact mAbs and their charge variants, as well as proteoform identification. The charge variants of NISTmAb were monitored across 45 injections using 3 different cartridges to evaluate the reproducibility of the icIEF-UV/MS workflow, Figure 1.
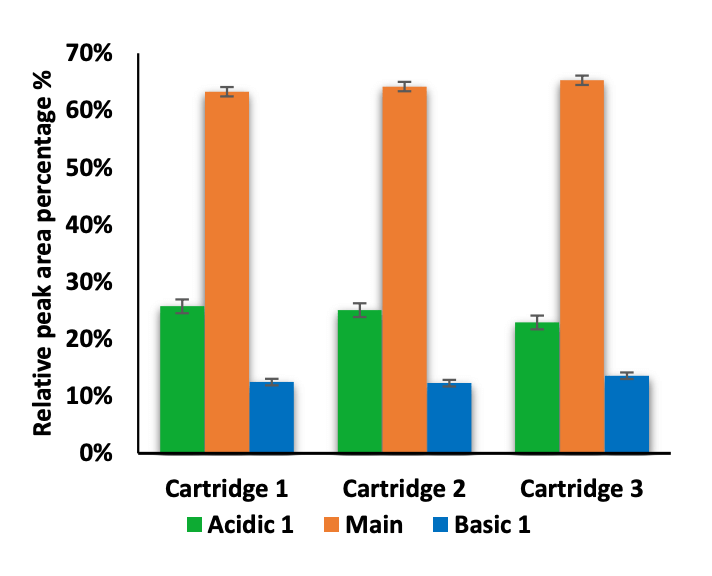
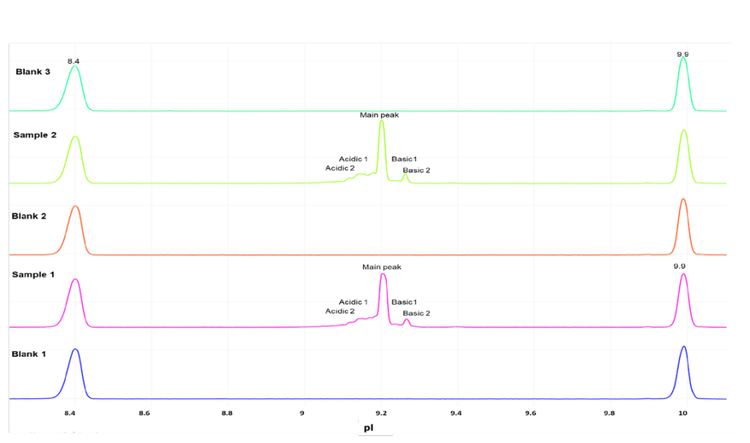
The intra- and inter-cartridge reproducibility of NISTmAb separation and quantitation were assessed based on 3 metrics. First, the relative percent abundances of the main, acidic 1, and basic 1 and basic 2 variants were determined using each of 3 different cartridges. Additionally, the icIEF-UV and icIEF-MS profiles acquired from the 3 cartridges were correlated and compared. Finally, the pI and peak separation were compared across multiple runs on the same cartridge.
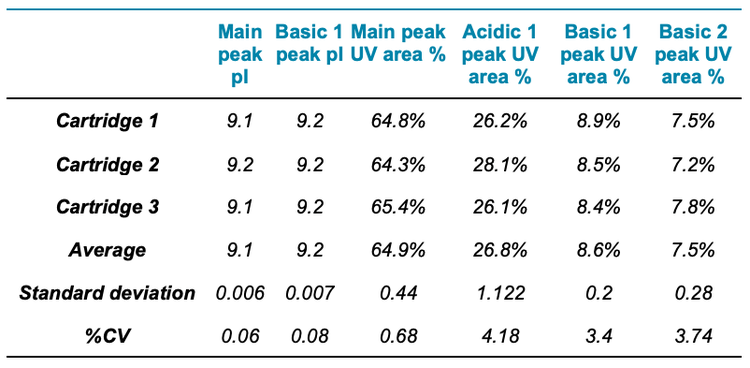
The icIEF-UV/MS analyses of NISTmAb using 3 different cartridges provided consistent pI values and relative abundances of the main, acidic1, basic 1 and basic 2 charge variants (Figure 2 and Table 3). The %CVs of the pI of the main peaks were <0.1% and the %CVs of the relative abundances of all variants based on the icIEF-UV profiles were <5% (Table 3). These results demonstrate the high inter-cartridge reproducibility of the icIEF-UV/MS workflow.
Intra-cartridge pI value and resolution reproducibility
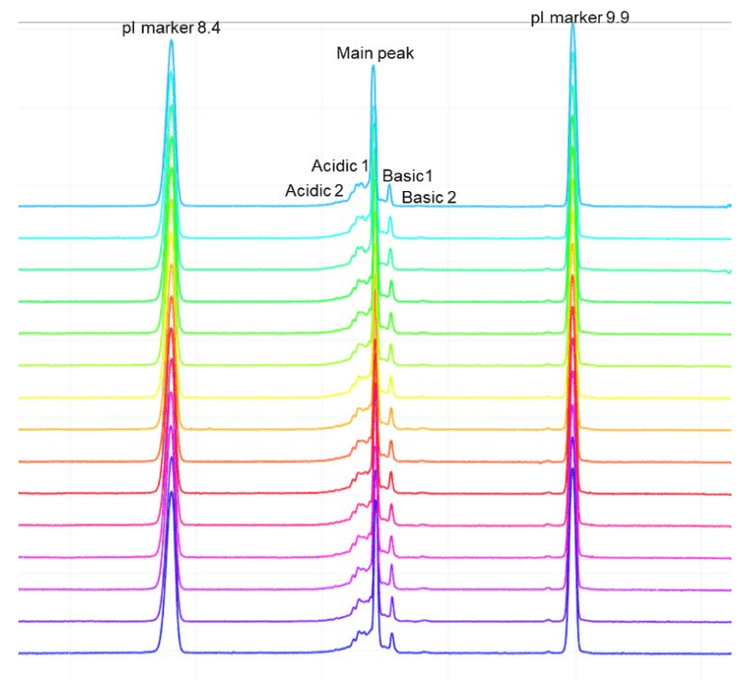
The icIEF-UV profiles were compared to evaluate the reproducibility of charge variant separation and detection across 15 injections on the same cartridge. Figure 3 shows the overlay of icIEF-UV profiles from 15 injections (blank injections are not shown).
Evaluation of carryover between runs
To assess the carryover between runs for the charge variant analysis, an alternating sequence of blank and highly concentrated NISTmAb (up to 1 mg/mL) samples was injected into the Intabio ZT system. The icIEF-UV profiles of the alternating blank and NISTmAb injections show that carryover was not detected in the blank runs between sample injections (Figure 5). These results demonstrate the absence of carry over in the described analysis on the Intabio ZT system.
Conclusion
- The Intabio ZT system is an innovative, chip-based integrated platform that offers high-resolution and highthroughput separation of intact mAbs and their charge variants
- The icIEF-UV/MS analysis demonstrates high reproducibility for both quantitation and resolution across 45 injections using 3 different cartridges
- The Intabio ZT system demonstrates a streamlined workflow to separate charge variants by icIEF and identify them with ZenoTOF 7600 system
- The Intabio ZT system is a robust platform that provides reproducible and high-resolution separation for intact protein analysis
- No carryover was observed for inter- and intra-cartridge injections on the Intabio ZT system
- The Intabio ZT system is a commercially available platform that offers workflow combining icIEF separation, UV quantitiation and MS-based identification.
- Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010 Aug;9(8):1716-28.
- Mack S, Arnold D, Bogdan G, et al. A novel microchip-based imaged CIEF-MS system for comprehensive characterization and identification of biopharmaceutical charge variants. Electrophoresis. 2019, 40: 3084-3091
- Ostrowski M. Raplid multi-atribute characterization of intact bispecific antibodies by a microfluidic chip-based integrated icIEF-MS technology. Electrophoresis. 2022 Oct;1-9.
- He X, ElNaggar M, Ostrowski MA, et al. Evaluation of an icIEF-MS platform for comparable charge variant analysis of biotherapeutics with rapid peak identification by mass spectrometry. Electrophoresis. 2022; 43: 1215– 22.

