Intermediate precision study of DNA analysis with the DNA 20 kb Plasmid and Linear kit on the BioPhase 8800 system
Fang Wang, Zylo Jacalne, Marcia Santos
SCIEX, USA
Abstract
This technical note highlights the intermediate precision of DNA analysis using the DNA 20kb Plasmid and Linear kit on the multi-capillary BioPhase 8800 system from SCIEX, covering both plasmid and linear double-stranded DNA (dsDNA). The kit overcomes the limitations of agarose gel electrophoresis and microchip-based capillary electrophoresis (CE). It provides a high-resolution, high-throughput, and reliable workflow to analyze plasmid topological isoforms distribution and linearized dsDNA size and purity.
The repeatability, reproducibility, and intermediate precision of the workflow were demonstrated using the plasmid test mix from the kit (Figure 1) and 1 kb Plus DNA Ladder with multiple cartridges (Figure 3), systems, and reagent lots. (Table 1)
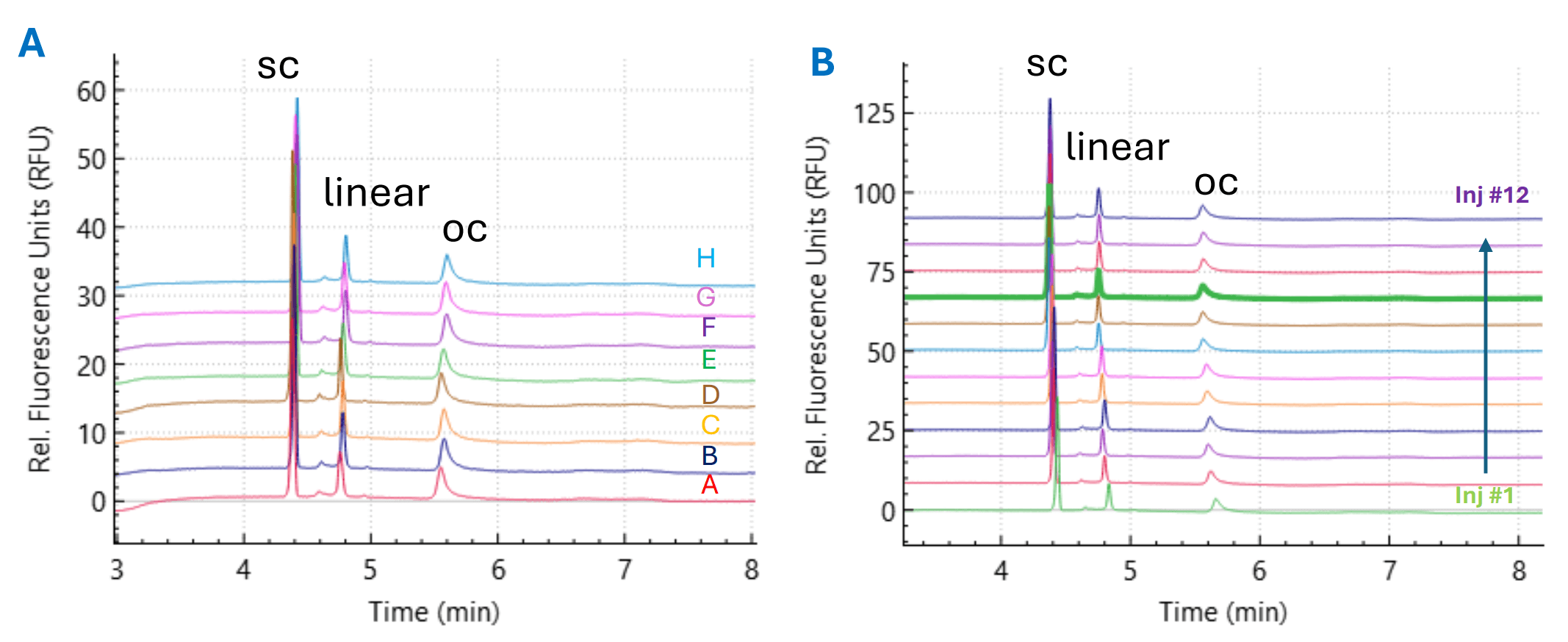
Figure 1. Representative electropherograms for plasmid topology analysis using the plasmid test mix (PTM, 5.4 kb) as a sample demonstrating the assay repeatability. Panel A shows overlay electropherograms of PTM in single injections across eight capillaries in a BioPhase BFS capillary cartridge - 8 × 30 cm. Panel B shows overlay electropherograms of PTM on a single capillary over 12 consecutive injections.
Key benefits of the DNA 20 kb Plasmid and Linear kit on the BioPhase 8800 system
- High-resolution separation of plasmid topological isoforms and linear dsDNA: This capability enables efficient analysis of plasmid purity and stability (2–19 kb) and linear DNA sizing and purity (0.1–20 kb).
- Great assay repeatability and reproducibility: When data from multiple cartridges, systems, analysts, and reagent lots are combined, the results show less than 1% relative standard deviation (RSD) for repeatability and less than 3% RSD for intermediate precision in both peak migration time and %purity of a sample for both plasmid and linear dsDNA purity analysis.
- One ready-to-use kit suitable for pre-built BFS cartridges for plasmid topology analysis and tunable linear DNA analysis: Streamlining the operations ensures consistent results and saves time and money.
Introduction
Plasmids are small, extrachromosomal circular DNA molecules capable of replicating autonomously, separate from nuclear DNA and independent of cell division. They have become critical materials in biopharmaceutical manufacturing and production. The global demand for plasmid DNA has surged significantly in recent years, driven by advancements in cell and gene therapy and nucleic acid vaccines.1 Consequently, the demand for analytical tools for characterizing and controlling the purity of plasmid samples has also increased.
Traditional agarose gel electrophoresis has limitations in resolution and automated quantitation. Microchip-based capillary electrophoresis (CE) faces challenges in obtaining high resolution between different plasmid topological isoforms. 2 The DNA 20 kb Plasmid and Linear kit on the BioPhase 8800 system offers a solution by providing fast and convenient analysis of both plasmid topological isoforms and linearized plasmid fragments. This work highlights the repeatability and reproducibility of the solution on the multi-capillary electrophoresis BioPhase 8800 system using a 5.4 kb plasmid test mix and a group of linear dsDNA ladders ranging from 0.1 to 20 kb as standard samples.
Methods
Materials: The DNA 20 kb Plasmid and Linear kit (P/N 5311708), the BioPhase BFS capillary cartridge - 8 x 30 cm (P/N 5080121), BioPhase BFS capillary cartridge - 8 x 50 cm (P/N 5080123), BioPhase sample and reagent plates (4,4,8) (P/N 5080311) were from SCIEX (Framingham, MA). The DNA 20 kb Plasmid and Linear kit contains DNA 20 kb Plasmid and Linear gel, DNA 20 kb Plasmid and Linear sample buffer, DNA 20 kb Plasmid test mix, SYBR™ Gold Nucleic Acid gel stain, DNA 20 kb Plasmid and Linear conditioning solution, Acid wash/regenerating solution and CE Grade water. 1 kb Plus DNA Ladder (P/N 10787018) was obtained from Thermo Fisher Scientific (Waltham, MA). All instrument methods were downloaded from the SCIEX website and used without modification for all the results shown here.3
Sample preparation: Plasmid test mix sample was prepared according to the kit application guide.3 Specifically, for each sample column (8 sample wells), one vial of the plasmid text mix was diluted with 800 µL DNA 20 kb Plasmid and Linear sample buffer. Linear dsDNA Ladder sample was prepared by diluting 5 µL of the 1 kb Plus DNA Ladder with 800 µL DNA 20 kb Plasmid and Linear sample buffer. Samples are mixed well using a vortex and aliquoted into the sample plates at 90 µL/well.
Reagent preparation: Separation gel was prepared according to the kit application guide. For every 20 mL of the DNA 20 kb Plasmid and Linear gel, 80 µL of SYBR™ Gold Nucleic Acid gel stain was incorporated. The specific volume of gel prepared is adjusted based on the reagent plate layout map generated by the BioPhase software for each sequence.
Instrument and software: The BioPhase 8800 system (P/N 5089278), equipped with a LIF detector (excitation 488 nm, emission 520 nm) controlled via BioPhase software, was used to perform all testing with the downloaded methods and to process all data.
Results and discussion for high-resolution plasmid analysis
The kit separates plasmids based on their topologies, resolving supercoiled (SC), linear, open circular (OC) and multiple aggregates of the SC species in one assay. This allows for the reporting of the purity of a given plasmid sample for process control. To evaluate the intermediate precision, the 5.4 kb plasmid was used as the representative sample, and three tests with various factors were performed as described in Table 1, generating results from 288 runs.
Table 1. Experimental design to access intermediate precision for plasmid purity analysis with a BioPhase BFS capillary cartridge - 8 × 30 cm.
Table 2. Plasmid analysis repeatability within a single test for intra- and inter-capillary variation
The plasmid test mix in the kit is an equal-ratio mixture of supercoiled plasmid and a controlled digestion sample of the same plasmid. The supercoiled plasmid’s main peak is reported as the purity, while all other species are combined and reported as impurity. The linear and OC peaks are used as representative impurity species to assess the repeatability in migration time.
Representative electropherogram MS of PTM in a single injection across eight capillaries (Figure 1A) and twelve consecutive injections on a single capillary (Figure 1B) are shown in Figure 1.
The assay repeatability was determined by calculating the average and %RSD for peak migration time and sample purity (%SC) within a capillary (N = 12) and for the entire data set within a test (N = 12 × 8 = 96), as shown in Table 2. The intra-capillary %RSD for peak migration is less than 0.6%, while the inter-capillary %RSD for peak migration time is no greater than 1.0%. The average %SC reported for all samples is about 58%, which is very close to the theoretical range of 50-60%, considering the yield of controlled digestion.
The intermediate precision for plasmid analysis was calculated by combining the results from all 288 runs. Critical values like peak migration time and %SC as sample purity were plotted in Figure 2, with the average and %RSD shown as an insert. The %RSD across three lots, with three system and cartridge combinations, is less than 3% for peak migration time and sample purity (%SC).
Figure 2. The intermediate precision for plasmid test mix (PTM) analysis using the DNA 20 kb Plasmid and Linear kit. Panel A: supercoil (SC), linear, and open circular (OC) peak migration time of all 288 runs with the average and %RSD shown in the inserted Table; Panel B: %SC as sample purity for all 288 runs with the average and %RSD shown in the inset.
Results and discussion for high-resolution linear dsDNA analysis
The same DNA 20 kb Plasmid and Linear kit can also be used for size-based linear dsDNA separation, achieving 5- 10% size resolution when using a BioPhase BFS capillary cartridge - 8 × 50 cm under the optimized separation conditions outlined in the kit application guide.3 The 1 kb Plus Linear dsDNA Ladder was used as the testing sample, containing 18 markers of different sizes ranging from 0.1 to 15 kb. The same experimental design shown in Table 1 was performed.
High-quality linear dsDNA samples generally have a minimal purity of 60%.4 In the sample, the % corrected area (%CA) for each marker is less than 10%, indicating these values are closer to the amount of impurity species in a sample than to the main peak. To evaluate the reliability of the assay in reporting linear DNA purity, markers sizing 0.1-1 kb are grouped and reported as lower markers (LM), and markers sizing 1.5 – 15 kb are grouped and reported as higher markers (HM). The intra- and inter-capillary average and %RSD for both groups is calculated and reported in Table 3.
Table 3. Linear dsDNA repeatability within a single test for intra- and inter-capillary variation.
Another common aspect of linear dsDNA analysis is size estimation, for which reproducible peak migration time is very important. The average and %RSD for migration time were calculated for all markers. Four of these markers— the smallest (0.1 kb), biggest (15 kb), and two additional markers in the lower (1.5 kb) and higher (6 kb) size ranges are shown in Table 4. All other markers (not shown) show very similar repeatability.
Table 4. Representative marker migration time (MT) repeatability within a single test for intra and inter-capillary variation in linear DNA analysis with a BioPhase BFS capillary cartridge - 8 × 50 cm.
Representative electropherograms of a single injection across eight capillaries (Figure 3A) and twelve consecutive injections on a single capillary (Figure 3B) are shown in Figure 3. These electropherograms demonstrate the assay repeatability and consistency in peak migration times across multiple capillaries and injections.
Figure 3. Representative electropherograms for linear dsDNA separated with a BioPhase BFS capillary cartridge - 8 × 50 cm using 1 kb Plus Linear DNA Ladder as a sample demonstrating the assay repeatability. Panel A shows overlay electropherograms of the1 kb Plus Linear DNA Ladder in single injections across eight capillaries in one cartridge. Panel B shows overlay electropherograms of the 1 kb Plus Linear DNA Ladder on a single capillary over 12 consecutive injections.
The intermediate precision for linear dsDNA analysis using a BioPhase BFS capillary cartridge - 8 × 50 cm was calculated by combining data from all 288 runs, as described in Table 1. The average and %RSD on migration time for all 18 markers, and the %CA for the two peak groups, were calculated and summarized in Table 5 and Table 6. The workflow demonstrates less than 2% RSD in migration time and %purity for DNA analysis.
Table 5. Intermediate precision for all 18 linear dsDNA markers using either a BioPhase BFS capillary cartridge - 8 × 50 cm or a BioPhase BFS capillary cartridge - 8 × 30 cm.
Table 6. Intermediate precision for %CA using either a BioPhase BFS capillary cartridge - 8 × 50 cm or a BioPhase BFS capillary cartridge - 8 × 30 cm.
Results and discussion for high-speed linear dsDNA analysis
While 5-10% size resolution (resolution no less than 1) can be achieved in the range of 0.1-15 kb by using a BioPhase BFS capillary cartridge - 8 × 50 cm with the DNA 20kb Plasmid and Linear kit, a 30 cm cartridge can also be used when higher speed is needed, and the impurity species have larger size differences from the main peak. Similar size estimate accuracy can be achieved by both workflows.7
The intermediate precision for linear DNA analysis on a BioPhase BFS capillary cartridge - 8 × 30 cm was evaluated following the experimental design shown in Table 7.
Table 7. Experimental design to access intermediate precision for linear dsDNA marker using a BioPhase BFS capillary cartridge - 8 × 30 cm.
Representative electropherograms of the 24 runs from a single test are shown in Figure 4. Lower peak resolution is observed, especially between 7 and 8 kb markers when compared with using a BioPhase BFS capillary cartridge - 8 × 50 cm (Figure 3). However, similar repeatability and reproducibility were observed. The results are shown in Table 8 and Table 9.
The intermediate precision for linear dsDNA analysis using a BioPhase BFS capillary cartridge - 8 × 30 cm was calculated by combining data from all 144 runs, as described in Table 7. The average and %RSD on migration time for all 18 markers, and the %CA for the two peak groups, were calculated and summarized in Table 5 and Table 6, along with the intermediate precision results for the BioPhase BFS capillary cartridge - 8 × 50 cm. The workflow demonstrates less than 3% RSD in migration time and less than 2% RSD in %purity for linear dsDNA.
Figure 4. Representative electropherograms for 1 kb Plus Linear dsDNA Ladder. The figure includes electropherograms in a single test with triplicate injections from 8 capillaries in a cartridge. Eighteen different markers ranging from 0.1-15 kb can be readily resolved.
Table 8. Intra- and inter-capillary repeatability for linear DNA marker migration time with BioPhase BFS capillary cartridge - 8 × 30 cm in a single test.
Table 9. Intra- and inter-capillary repeatability for linear DNA marker group peak %CA with BioPhase BFS capillary cartridge - 8 × 30 cm in a single test.
Conclusion
- The remarkable assay repeatability using the DNA 20 kb Plasmid and Linear kit for plasmid and linear dsDNA analysis was demonstrated.
- One versatile kit can be used for both plasmid topology analysis and linear dsDNA size-based analysis on the multi-capillary BioPhase 8800 system. Two different cartridge options allow tunable peak resolution, making it easy to develop fit-for-purpose analytical methods. Improved size resolution can be achieved when using the BioPhase BFS capillary cartridge - 8 × 50 cm while maintaining similar repeatability
References
- Jonny Ohlson (2020), Plasmid manufacture is the bottleneck of the genetic medicine revolution. Drug Discov Today. 2020 25(11): 1891–1893.
- Li Ding, Kathi Williams, Walter Ausserer, Luc Bousse, Robert Dubrow (2003) Analysis of plasmid samples on a microchip. Anal Biochem. 2003 316(1):92-102. PMID: 12694731.
- DNA 20 kb Plasmid and Linear kit for the BioPhase 8800 system. SCIEX application guide, RUO-IDV-05- 15737-A.
- FDA. Guidance for Industry: Considerations for Plasmid DNA Vaccines for Infectious Disease Indications. 2007 November.
- Li Ding, Kathi Williams, Walter Ausserer, Luc Bousse, Robert Dubrow (2003) Analysis of plasmid samples on a microchip. Anal Biochem. 2003 316(1):92-102. PMID: 12694731.
- Unlocking the full potential of the DNA 20 kb Plasmid and Linear kit for comprehensive plasmid topology and linear DNA sizing analysis. SCIEX technical note, MKT32622-A
 Click to enlarge
Click to enlarge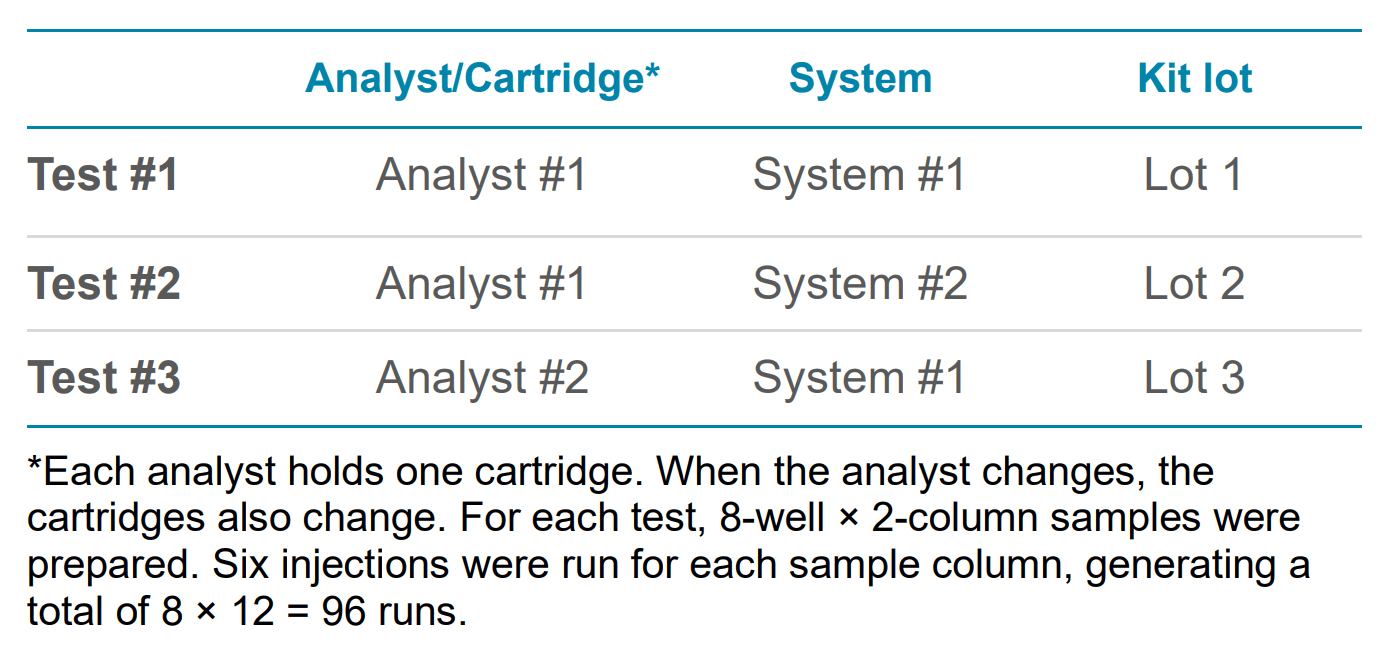 Click to enlarge
Click to enlarge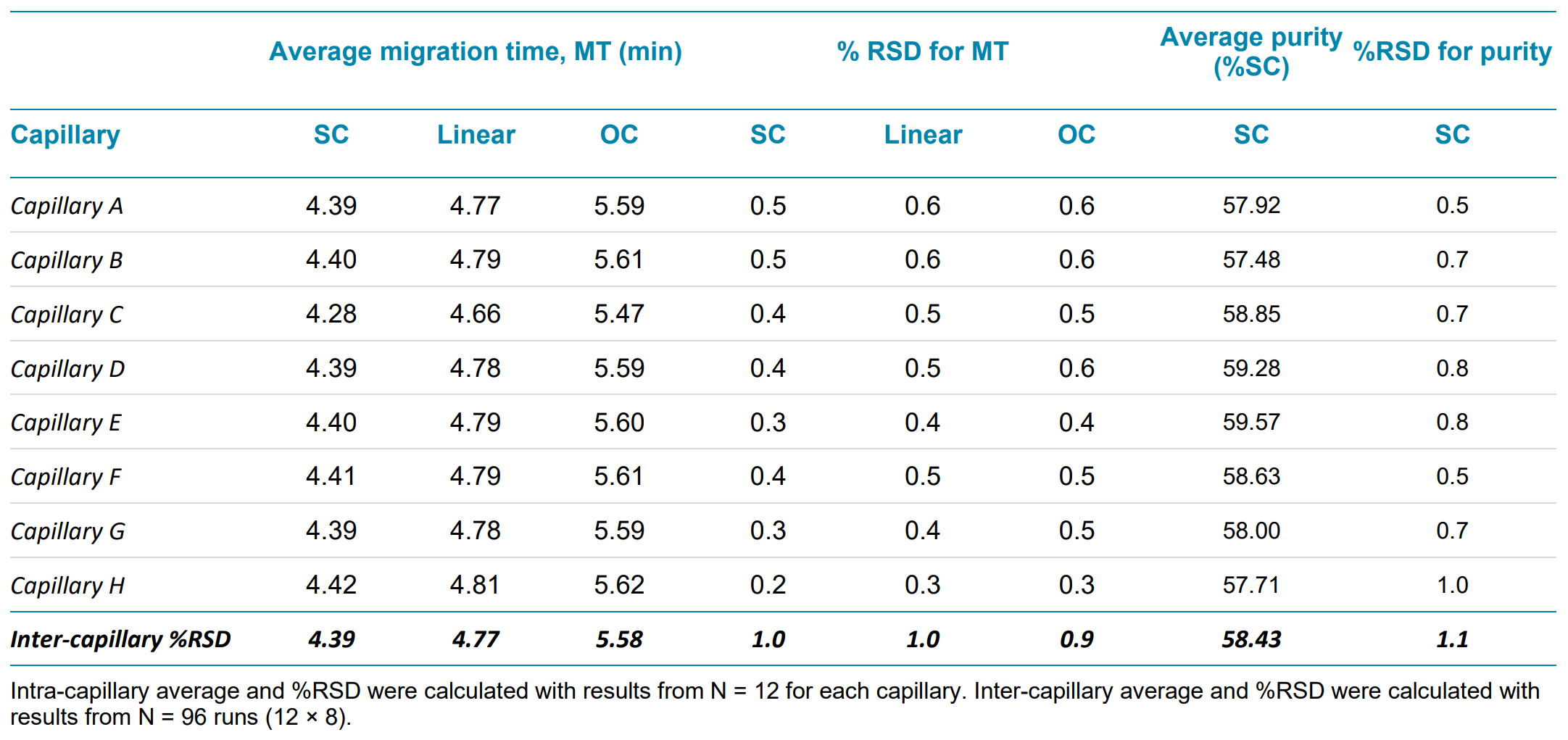 Click to enlarge
Click to enlarge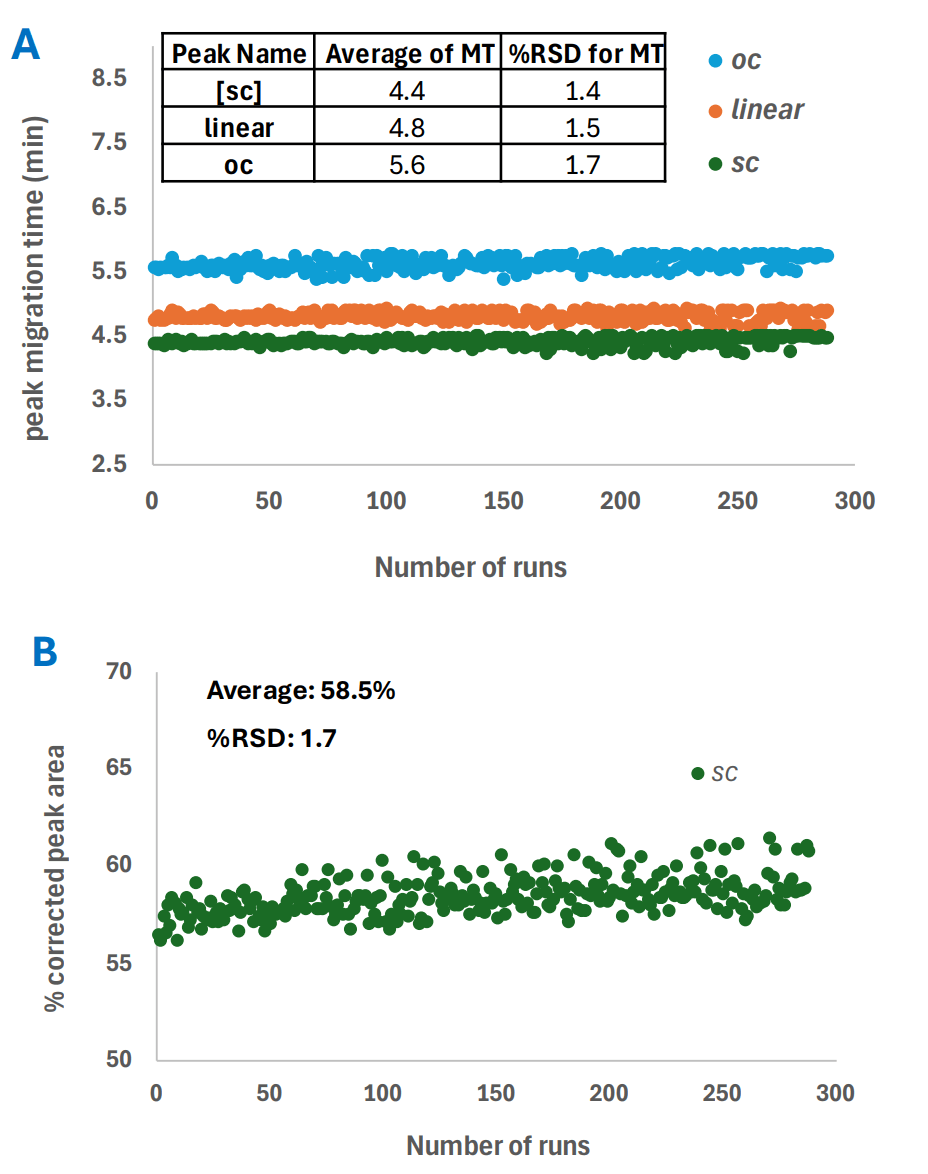 Click to enlarge
Click to enlarge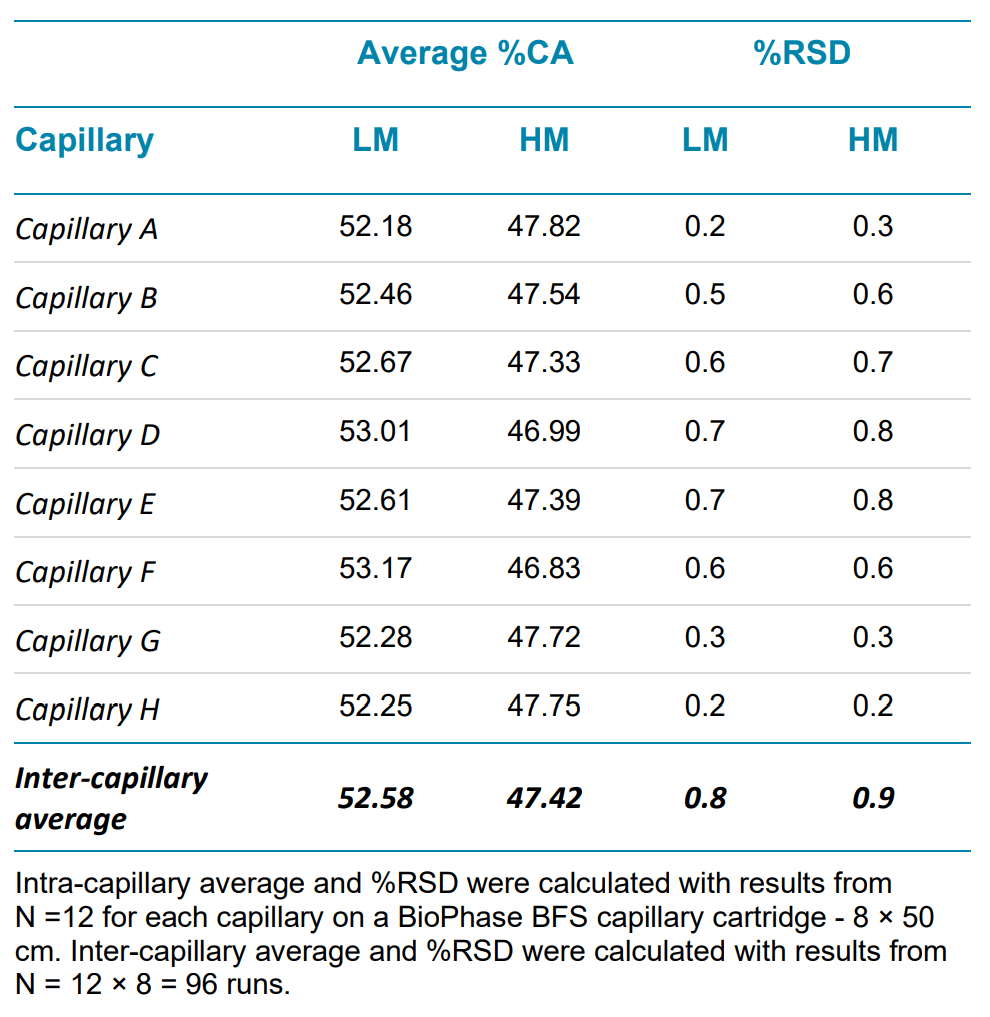 Click to enlarge
Click to enlarge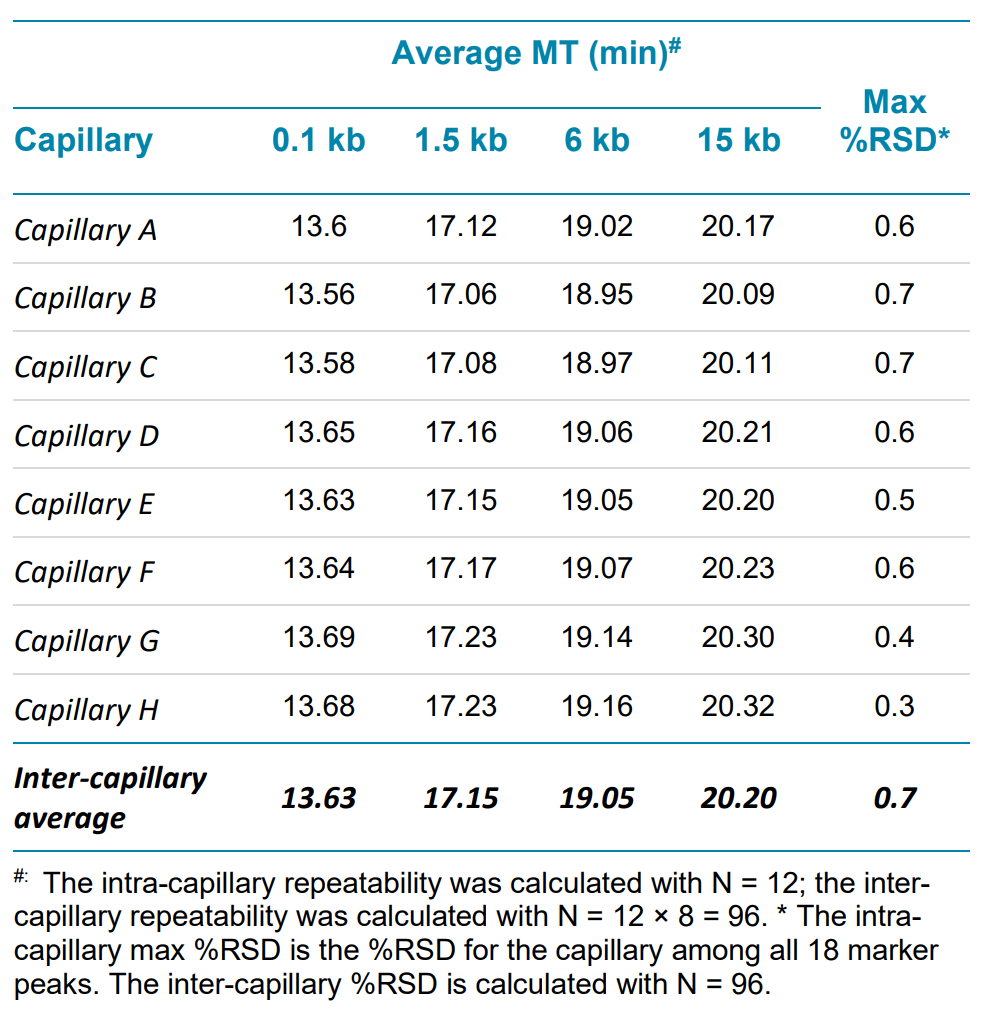 Click to enlarge
Click to enlarge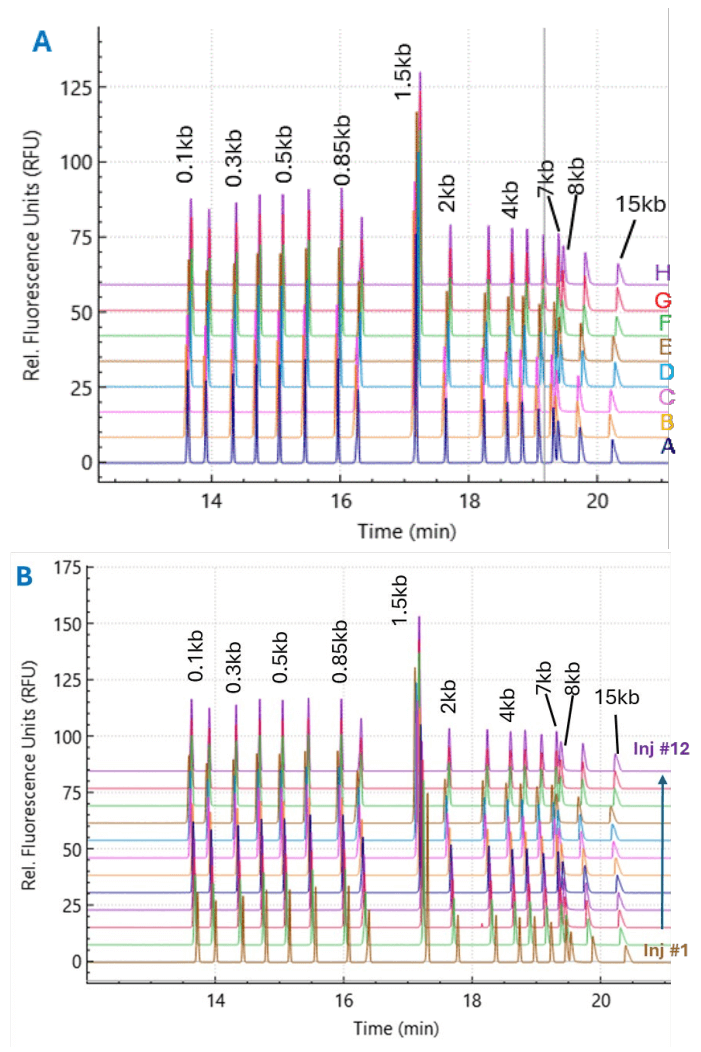 Click to enlarge
Click to enlarge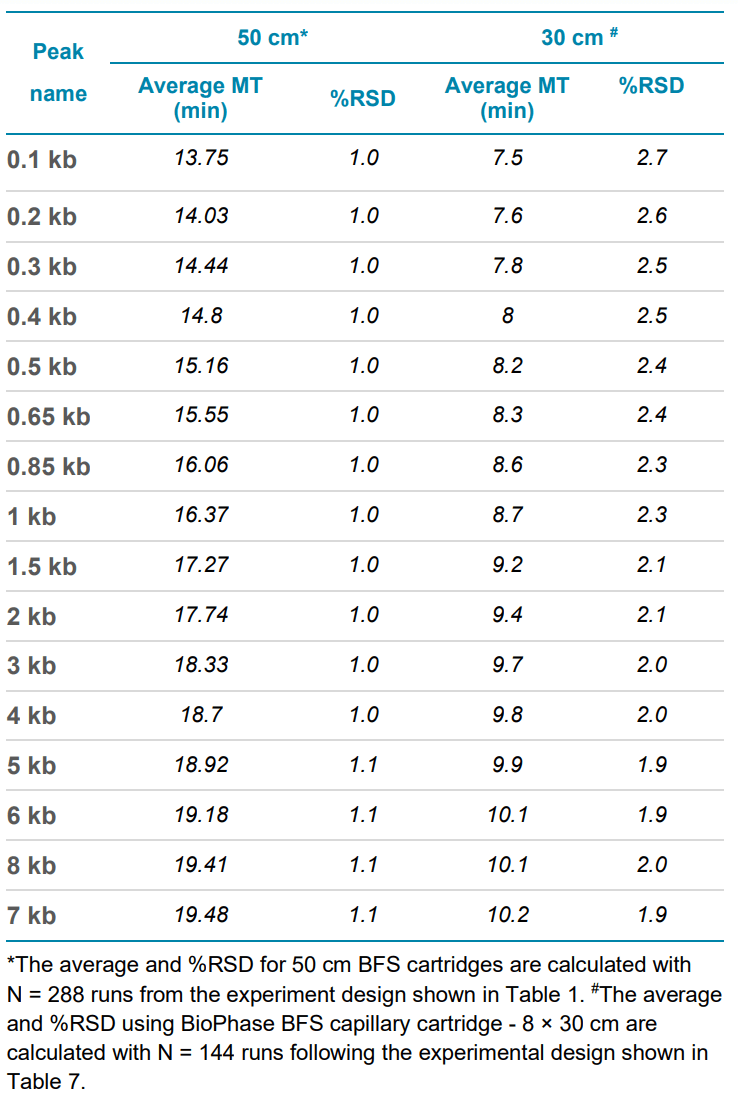 Click to enlarge
Click to enlarge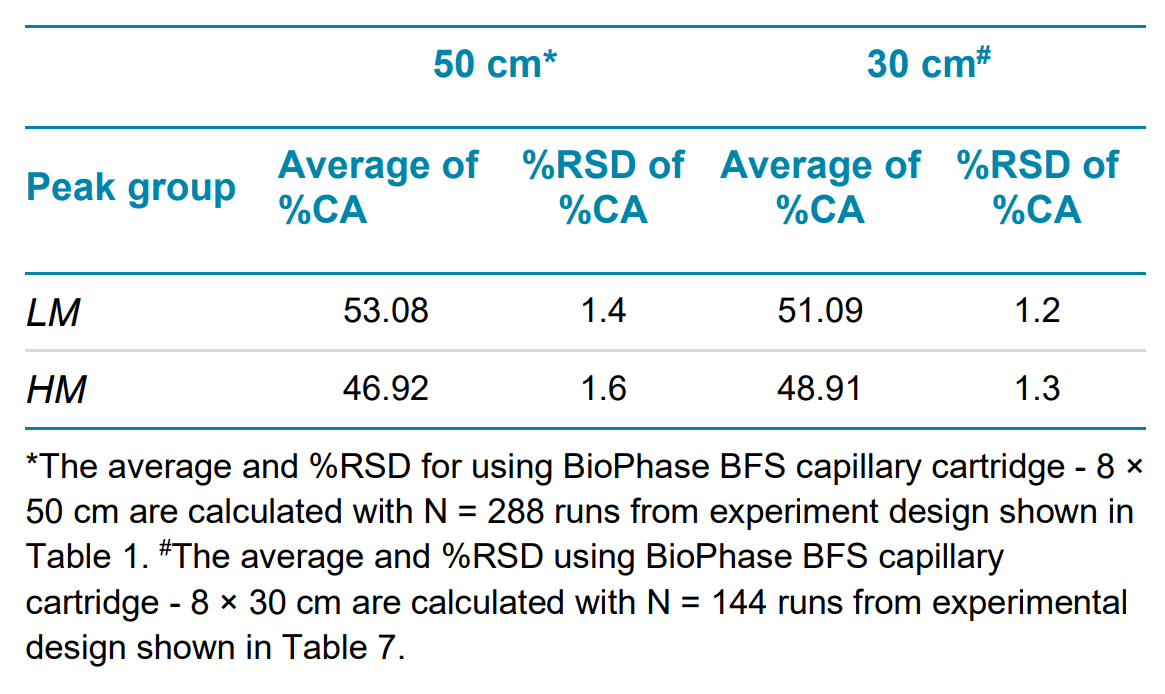 Click to enlarge
Click to enlarge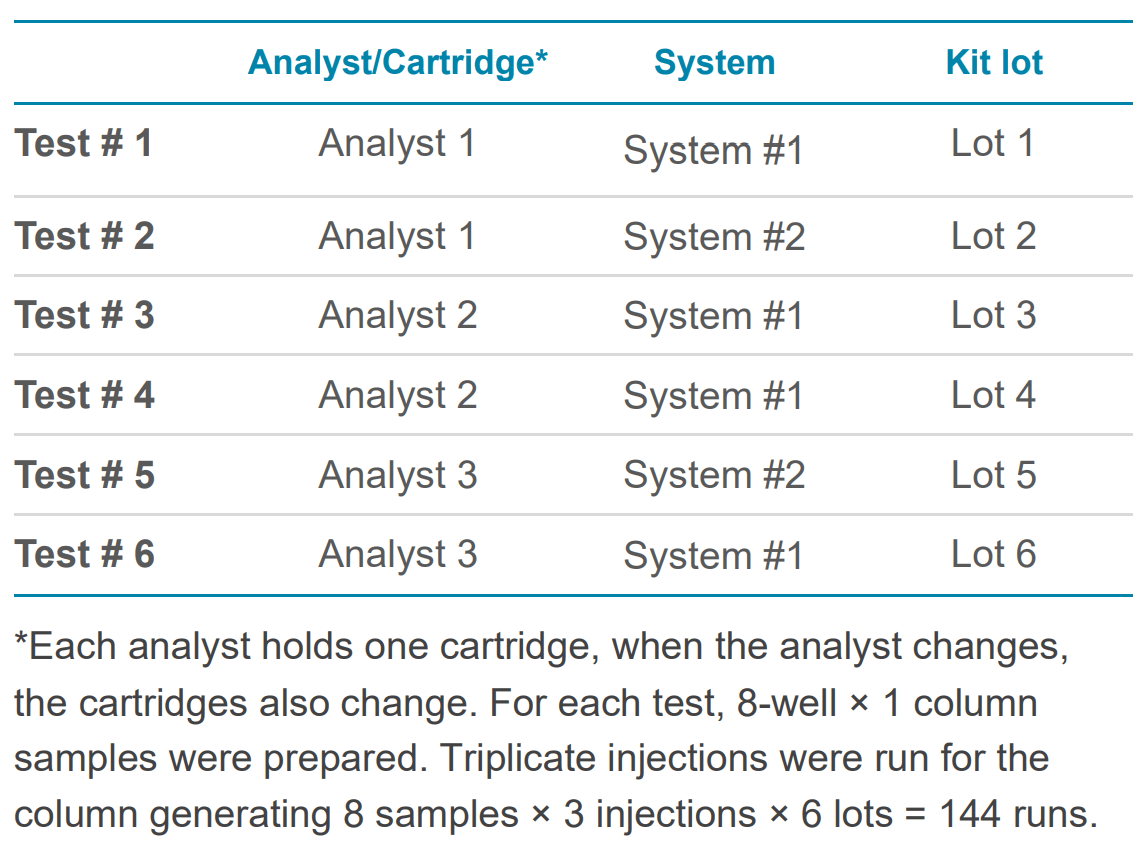 Click to enlarge
Click to enlarge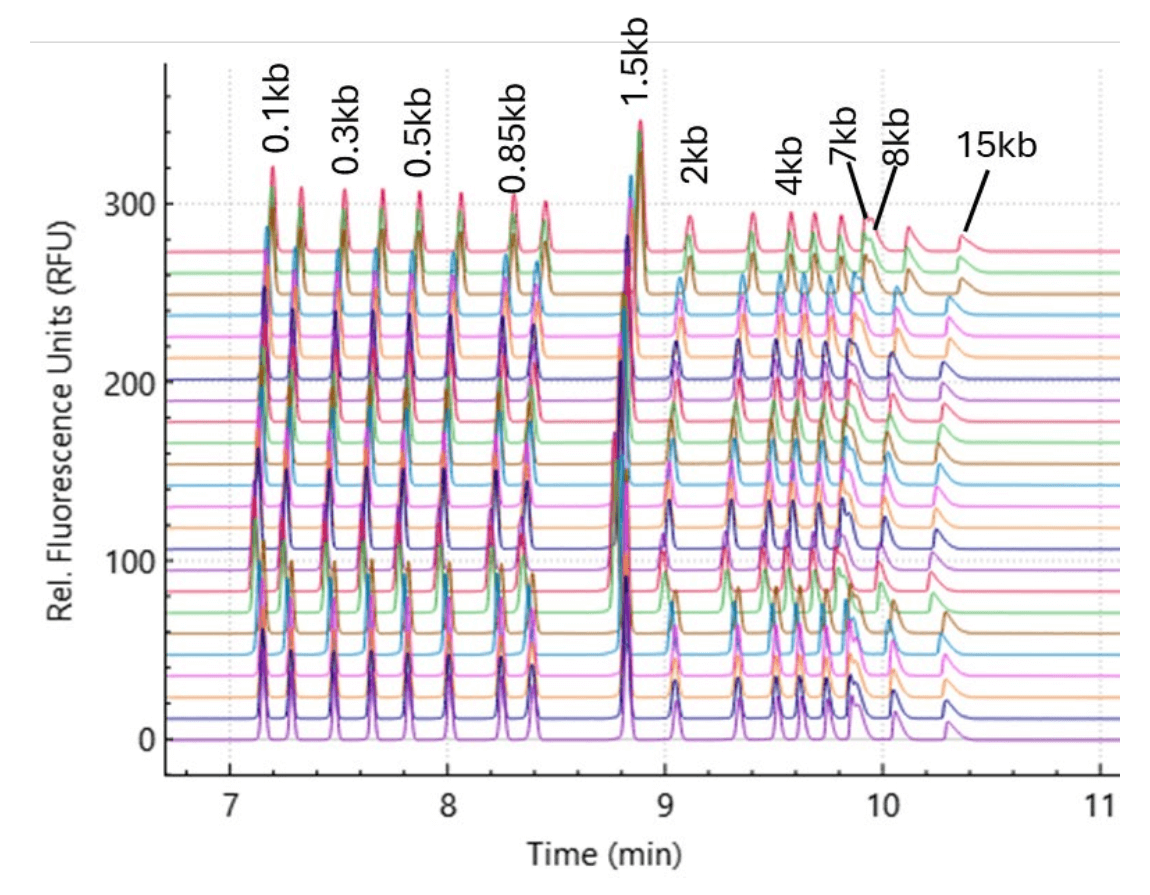 Click to enlarge
Click to enlarge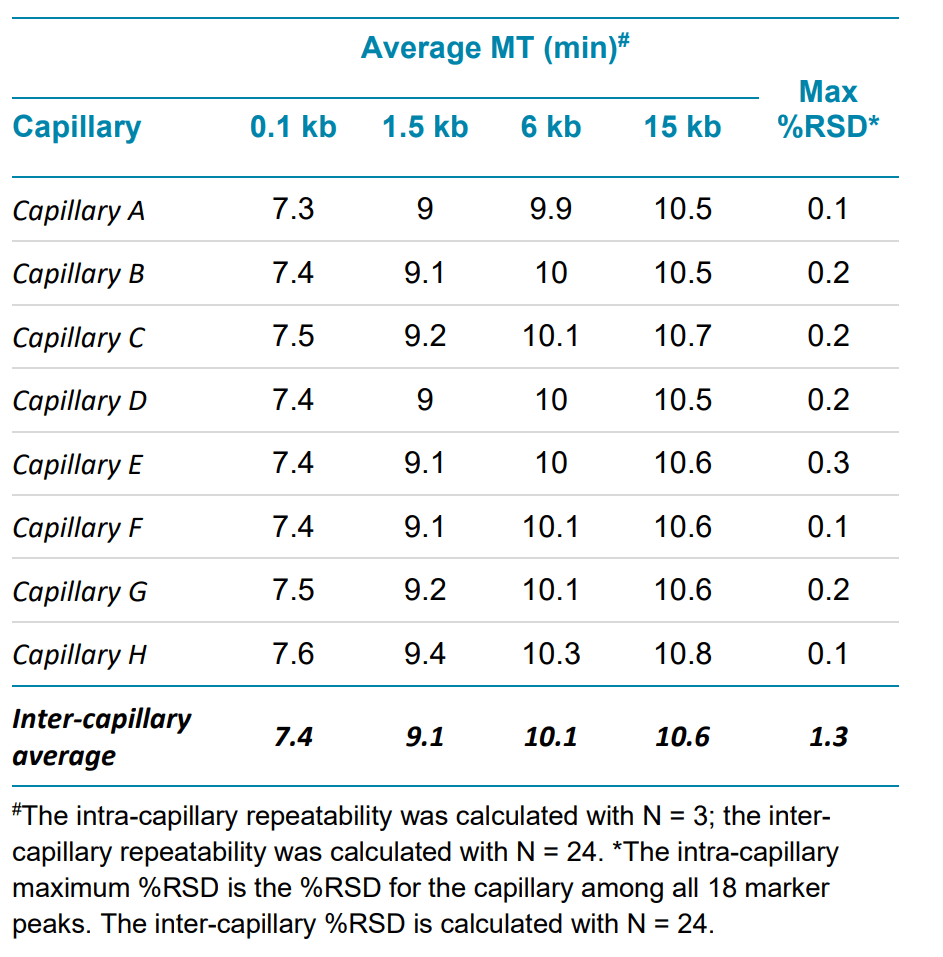 Click to enlarge
Click to enlarge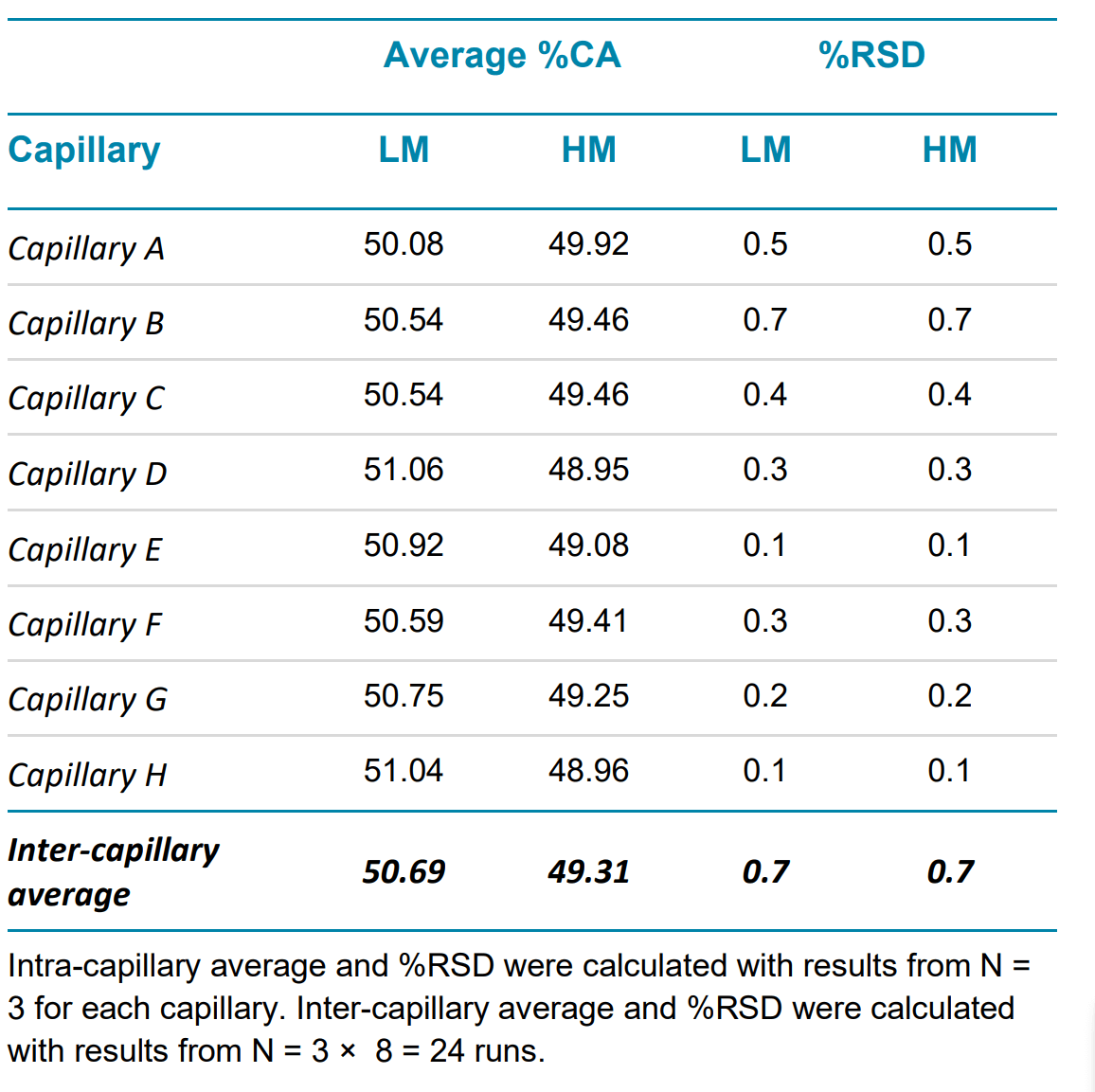 Click to enlarge
Click to enlarge