Abstract
The purity of AAV capsid proteins has important implications for efficacy of the viral vector.1 A robust analytical method for assessment of the capsid purity for a range of serotypes that can provide reliable results in a timely fashion is desirable. Multiple factors such as temperature and buffer concentrations affect the capsid protein stability which impacts the assembly of the capsids. Optimization of these parameters are needed in order to perform accurate evaluations. However, the analysis time for optimization of each parameter can be time consuming. This technical note demonstrates how the recently introduced SCIEX BioPhase 8800 multi-capillary electrophoresis system can be used to significantly reduce time for method optimization and DOE studies by multiplexing analysis on a single platform.
Introduction
The purity of AAV capsid proteins has important implications for efficacy of the viral vector.1 A robust analytical method for assessment of the capsid purity for a range of serotypes that can provide reliable results in a timely fashion is desirable. Multiple factors such as temperature and buffer concentrations affect the capsid protein stability which impacts the assembly of the capsids. Optimization of these parameters are needed in order to perform accurate evaluations. However, the analysis time for optimization of each parameter can be time consuming. This technical note demonstrates how the SCIEX BioPhase 8800 system can be used to significantly reduce time for method optimization by a one multi-capillary electrophoresis system CE-SDS (capillary electrophoresis sodium dodecyl sulfate) technology is popular for protein analysis in the biopharmaceutical industry because of its automated, quantitative protein separation capability. It is high resolution, reproducible and less laborious than traditional SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis).2

CE-SDS has been successfully used for separation of the various capsid proteins and the results are consistent across serotypes.3,4 Method optimization for those studies was done using a single capillary format, which can limit throughput.
This technical note highlights the use of the BioPhase 8800 system for accelerating method development for AAV. The ability to perform 8 separations in parallel allows for faster processing of samples and overall increased throughput.
Key features
- Optimization of 3 separate parameters takes 4 hours to complete on the multi-capillary BioPhase 8800 system compared to 48 hours on the single capillary PA 800 Plus.
- Easy-to-use new processing software provides flexibility in viewing and reporting of data results.
- Optimized parameter settings obtained on the BioPhase 8800 system correlate well with values on the PA 800 Plus.
- Efficient labeling by Chromeo dye P503 and a simple sample preparation procedure completed in less than 1 hour.
Materials and methods
Materials
Chemicals: The SDS-MW Analysis Assay kit (Part # 390953, SCIEX, Brea, CA) with the SDS-MW gel buffer and sample buffer were from SCIEX (Framingham, MA, USA). The Chromeo P503 dye (PN 15106) was from ACTIVE MOTIF (Carlsbad, CA, USA). Sodium dodecyl sulfate (PN L4390-100G), and 2-mercaptoethanol (PN M3148-100ML), N-Ethylmaleimide (PN 04259), DL-Dithiothreitol (PN D9163), and all other chemicals were from Sigma Aldrich (St. Louis, MO, USA).
Sample preparation for AAV CE-SDS-UV
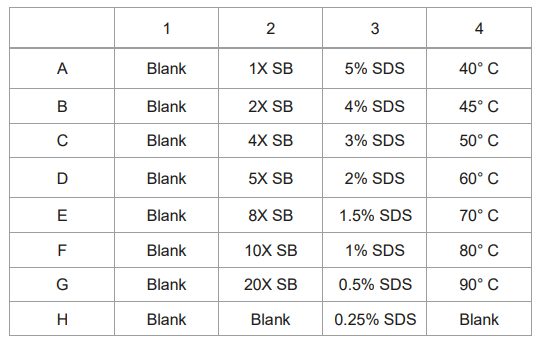
The method is developed and optimized using AAV8 samples. For the optimization of sample preparation procedure, 5 µL of AAV8 sample solution was mixed with 5 µL of incubation buffer and 1.5 µL of 2-mercaptoethanol in a 0.65 mL micro-centrifuge tube at a constant temperature for 10min. Samples were then allowed to return to room temperature before DI water was added to the mixture. The diluted mixture was transferred into the appropriate well of the injection sample inlet plate for analysis on the BioPhase 8800 system. Different incubation buffers and incubation temperatures were evaluated to achieve the optimal sensitivity and minimum sample preparation. 1X, 2X, 4X, 5X, 8X,10X, and 20X dilutions of sample buffer from the SDS-MW kit (100 mM Tris-HCl pH 9.0, 1% SDS) and SDS solutions at different concentrations from 0.01% to 1% were compared in this technical note. The incubation temperature ranging from 40° C to 90° C were also evaluated for the optimal sample preparation conditions.
Sample preparation for AAV CE-SDS-LIF BioPhase
20 µL of AAV sample, diluted as previously described, was mixed with 20 µL of Tris sample buffer, 4 µL of 1M DTT, and 1 µL of 2 mg/mL Chromeo P503 dye5 and incubated at 60° C for 20 min. After cooling the samples down to room temperature, 154 of DI water was added to the mixture. AAV1, AAV2, and AAV8 were prepared following the sample preparation procedure. 100 µL of the diluted, prepared sample solution was transferred into the well of the injection sample inlet plate for analysis on the BioPhase 8800 system. The leftover 100 µL of the diluted prepared sample solution was transferred to the sample vial for analysis on the PA 800 Plus.
All single capillary electrophoresis analyses were accomplished using a PA 800 Plus Pharmaceutical Analysis system configured with LIF detector and solid-state laser with excitation wavelength that 488 nm and a 600 nm band pass emission filter from Edmund Optics (Barrington, NJ). CE-SDS separations were performed using the EZ-CE cartridge (Part # A55625) with a 20 cm effective length (30 cm total length). A 50 µm ID bare fused silica capillary was filled with the SDS-MW gel-buffer system. Capillary conditioning was: 0.1 M NaOH rinse for 3 minutes at 70 psi, 0.1 M HCl rinse for 1 minute at 70 psi, HPLC grade water rinse for 1 minute at 70 psi and SDS-MW gel buffer rinse for 10 minutes at 80 psi before each run. The applied electric field strength was 500 V/m for all capillary electrophoresis analyses in reversed polarity mode (anode at the detection side). The samples were electrokinetically injected at 5 kV for 20 seconds. The 32 Karat software 10.1 package was used for data acquisition and processing (Part # A79486AC, SCIEX).
The multiplexed separation utilized the BioPhase 8800 system. The gel-buffer system, capillary conditioning, injection, and separation conditions (Figure 6) were the same as those for the single capillary analyses. Separations were accomplished in the BioPhase BFS Capillary Cartridge – 8 x 30 cm. The BioPhase software package was used for data
Results and discussion
Sample buffer optimization
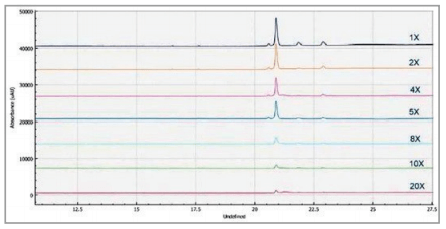
Different sample preparations and buffers were evaluated to achieve optimal sensitivity and resolution of the capsid proteins for the AAV 8 serotype on the BioPhase 8800 system using CE-SDS-UV. One of the parameters optimized was the sample buffer. Sample buffer from the SDS-MW kit (100mM Tris-HCL, pH9.0, 1% SDS) was used at 1x-20x dilution range as shown in Figure 5. Data from all7 dilution points were obtained from a 30 min single analysis on the multi-capillary. The analysis shows the best peak intensity was obtained using 1x sample buffer which correlates well with results obtained from previous studies on the PA 800 Plus.3
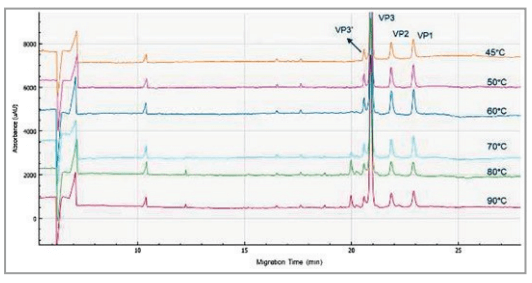
Incubation temperature optimization
The optimization of the sample incubation temperature for the AAV8 serotype is shown in Figure 1. The peak intensity of all 3 capsids are optimized at 50° C. With increased incubation temperature greater than 50° C, smaller peaks with increased intensity is observed while the VP3 protein peak intensity decreases. These smaller peaks are likely degradation products of the VP3 proteins. This method is optimized for the AAV 8 sample, the optimal incubation temperature can differ for different serotypes.
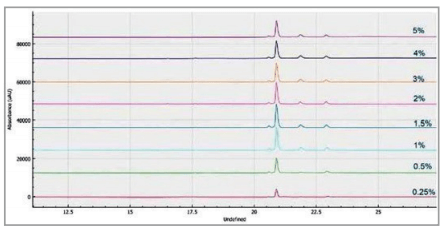
% SDS optimization
Figure 6 shows the optimization of the % SDS concentration used for the optimal method. 5 L of 1% - 1.5% SDS provided the optimal peak shape and sensitivity. This provides sufficient amounts for protein binding and minimal residual salt concentration for best efficiency of electrokinetic sample injection.
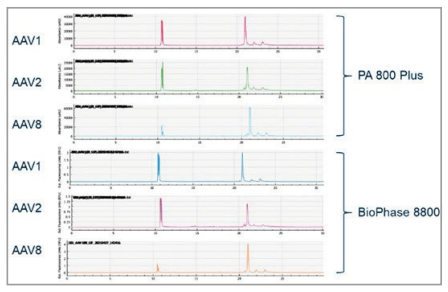
Comparison between BioPhase 8800 and PA 800 Plus
To compare the data quality between the multi-capillary and the single capillary platforms, analysis of 3 different AAV serotypes, 1, 2, and 8, respectively was performed on both the PA800 Plus and BioPhase 8800 systems. The sensitivity and migration time of the 3 capsid proteins align well between the 2 systems (Figure 7). Additionally, as expected the migration times obtained for each serotype also correlated well between the 2 systems.
Conclusions
- Multiplexing capability of the BioPhase 8800 allows for 12x faster analysis compared to the single capillary systems for the method optimization of AAV purity analysis by CE-SDS.
- The sample preparation with the Chromeo P503 dye labeling for LIF analysis was simple and did not require any buffer exchange of sample cleanup. This helps in decreasing time for sample preparation and overall workflow completion time.
- Optimized conditions obtained on the multi-capillary system correlates well with the values obtained on the PA 800 Plus system. Thus, providing a seamless and easy method transfer from the single to the multi-capillary platform.content here
References
- Kewal, J.K.; Drug Delivery Systems,.2008; 437: 51–91
- Ying, Shi.; Zen, Li.; Li, Jun; Anal. Methods, 2012,4, 1637-1642
- Purity Analysis of Adeno- Associated Virus (AAV) Capsid Proteins using CE-SDS method, RUO-MKT-02-9761-A
- Zhang, C.; Meagher, M. M. Anal. Chem. 2017,
- Sensitive AAV capsid protein impurity analysis by CE using easy to label fluorescent Chromeo dye P503, RUO-MKT-02-10600-A
