Detection of xylazine in whole blood samples using highresolution mass spectrometry
Abstract
This technical note describes a non-target acquisition method for the simultaneous screening and quantitation of the emerging drug xylazine in authentic human whole blood sample. Using the SCIEX X500R system, the MS/MS spectra were searched against a user-generated library database for compound confirmation. This screening method revealed detectable levels of xylazine in 4 of 7 authentic whole blood samples suspected to contain fentanyl and/or other opioids (Figure 1). The screening method also revealed the presence of additional drugs in the blood samples.
Introduction
In recent years, there has been a growing concern about the increasing use of xylazine as an adulterant in illicit drug mixtures.1 This rise in the prevalence of xylazine has also coincided with an uptick in overdose deaths.1 Originally designed for veterinary purposes as a tranquilizer and anesthetic, xylazine is not classified as a controlled substance under the Controlled Substances Act and is not approved for human use.1
Poly-drug intake, such as the addition of xylazine to synthetic opioids, such as fentanyl, significantly increases the risk of overdose. Individuals using illicit drugs might not be aware of the presence of non-opioid substances, such as xylazine, in drug products. Given the concerns about the role of xylazine in opioid overdoses, it is essential to employ a comprehensive drug screening approach. SWATH DIA is a valuable tool for this purpose as it collects MS/MS fragment data on all features present in a sample. This untargeted screening approach provides information to aid in identifying and quantifying known and emerging drugs and adulterants in toxicology samples.
Key benefits of the X500R system and SWATH DIA for the screening and quantitation of xylazine
- SWATH DIA acquires MS/MS data on all features, creating a digital record of the sample for retrospective screening
- The screened panel consisted of a diverse group of drug classes, including stimulants, opioids, benzodiazepines and antidepressants
- Excellent sensitivity was demonstrated with simultaneous identification and quantitation of xylazine at levels as low as 1 ng/mL in the presence of other opioids, stimulants, and illicit drugs in human whole blood
Methods
Sample preparation: Authentic human whole blood samples were aliquoted (0.5 mL), pretreated with 3 mL of phosphate buffer (pH 6) and centrifuged at 3000 rpm for 10 minutes. The samples were loaded onto a solid phase extraction (SPE) cartridge consisting of a C8 and ion exchange phase (benzenesulfonic acid) bonded to the same particle (CleanScreen, United Chemical Technologies, PA). Cartridges were conditioned with methanol and water, followed by equilibration with 1 mL of phosphate buffer. After sample loading, the cartridge was washed with water, followed by 0.1M HCl, then methanol, before drying under vacuum. The analytes were eluted using 2 aliquots of 1.5 mL of 78:20:2 dichloromethane/isopropyl alcohol/ammonium hydroxide. Finally, 100 µL of 90:10 methanol/HCl solution was added, and the sample was dried under a nitrogen stream and reconstituted to 200 µL in 95:5 water/acetonitrile.
Calibrator preparation: A 10 ng/µL stock standard solution mixture containing the target drugs was prepared via dilution of 1 mg/mL stock standards with methanol (Table 1). Calibration solutions were prepared using the 10 ng/µL stock standard solution mixture to evaluate the dynamic range. These, in turn, were used to spike 500 µL of blank human whole blood to prepare a matrix-matched calibration series covering concentrations ranging from 1 ng/mL to 100 ng/mL. Finally, 25 µL of the 1 ng/µL IS spiking stock solution was added to each calibration level.
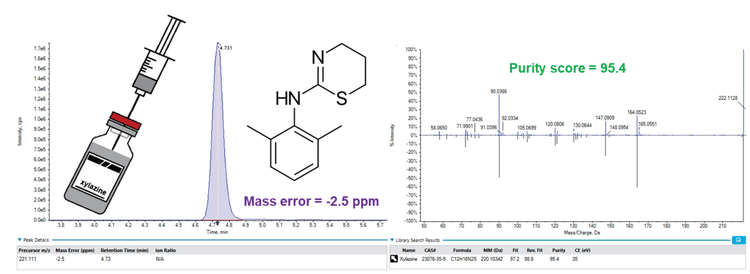
Chromatography: The injected sample volume was 10 µL. Analytical separation was performed using a Phenomenex Kinetex Core-shell C18 column (50 mm x 3 mm, 2.6µm, Part No: 00B-4462-Y0). Mobile phase A consisted of water with 10mM ammonium formate at pH 3 with formic acid, while mobile phase B was 50:50 acetonitrile/methanol. Mobile phase B was initially held at 5% for 1 minute and then ramped to 95% within 9 minutes, where it was held for 3 minutes. The total run time was 15.5 minutes (Figure 2).
Mass spectrometry: MS and MS/MS data were collected using SWATH DIA on the SCIEX X500R QTOF system with SCIEX OS Software 3.3, each SWATH DIA scan beginning with a TOF MS experiment. Variable SWATH DIA precursor window widths were used throughout the mass range, as shown in Figure 3. The method source conditions included curtain gas set to 30 psi, ion source gas 1 set to 50 psi, source gas 2 set to 50 psi, ion spray voltage set to 2500 V, and ion source temperature set to 600°C. MS/MS fragmentation was acquired using SWATH DIA with a collision energy of 35 V and a collision energy spread of ± 15 V.
Data processing: SCIEX OS software 3.3 was used for library screening and quantitation. Features were screened against the Center for Forensic Science Research & Education (CFSRE) custom spectral library. Compounds were considered detected if the mass error was <5 ppm, library fit score >70, and retention time <2.5% of the calibration standards. Analytical standards were then analyzed and used for confirmation and quantitation.
Case study: Authentic human whole blood screening and quantitation using SWATH DIA acquisition
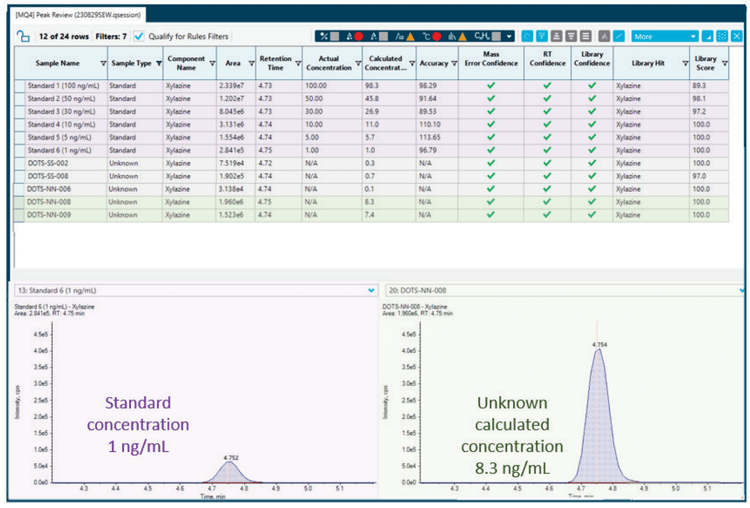
A calibration curve containing a panel of 10 drugs, including xylazine, at concentrations ranging from 1 to 1000 ng/mL, was used for the quantitation of unknown whole blood samples. The lower limit of quantitation (LLOQ) was required to have an accuracy within ± 20% and a signal-to-noise ratio (S/N) greater than 10. For all 10 compounds, the LLOQ was successfully established at 1 ng/mL, which is sufficient for forensic screening methods.
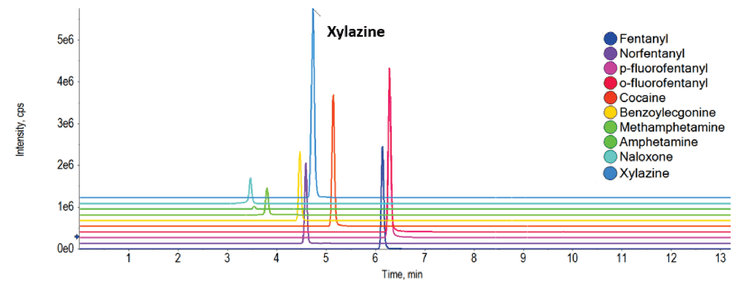
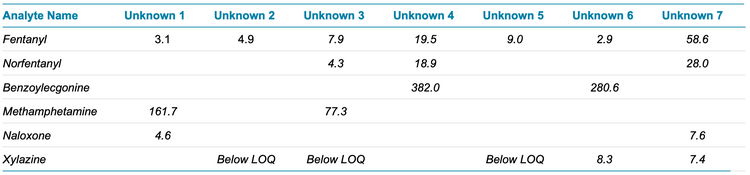
In our screening process, a total of seven whole blood samples known to contain fentanyl were analyzed. Notably, two of these samples contained xylazine at concentrations above the LLOQ, measuring 7.4 ng/mL and 8.3 ng/mL (Figure 4 and Table 1). Additionally, three samples exhibited detectable levels of xylazine but were found to be below the LLOQ. As shown in Figure 4, xylazine detection is quickly flagged in the SCIEX OS results table using the criteria of mass confidence, RT confidence and library confidence (green check marks). These criteria can also be filtered by the user allowing the positive hits to be rapidly highlighted in large data sets.
It is worth highlighting that none of the seven samples contained solely fentanyl but exhibited the presence of 2 to 3 additional drugs and/or metabolites from the panel. This underscores the complexity and potential poly-drug use associated with the source of these samples, demonstrating the importance of comprehensive screening and quantitation techniques in such analytical contexts.
Given that the data was acquired through a non-targeted SWATH DIA approach, the processing method developed in this study for the targeted screening of specific compounds can be readily adapted to identify unknown compounds. It is possible to conduct retrospective analyses of previously obtained data sets, making it easier to detect novel compounds, all while avoiding the need to reinject samples. Data sets can be systematically reprocessed quickly in SCIEX OS if new forensic targets are identified for quantitation, as demonstrated in this study. This highlights the flexibility and adaptability of the SCIEX OS software.
Conclusion
- A robust method was developed for the simultaneous screening and quantitation of xylazine in authentic human whole blood samples known to contain fentanyl
- LLOQ of 1 ng/mL for all compounds was shown in the matrix-matched calibration standards
- The screening of seven whole blood samples containing fentanyl identified xylazine above the LLOQ in two samples and detected it in three others.
- None of these samples contained solely fentanyl, emphasizing the complex and diverse exposure observed in poly-drug use scenarios.
References
- The Growing Threat of Xylazine and its Mixture with Illicit Drugs: DEA Joint Intelligence Report; US Drug Enforcement Administration (October 2022)
https://www.dea.gov/sites/default/files/2022- 12/The%20Growing%20Threat%20of%20Xylazine%20and %20its%20Mixture%20with%20Illicit%20Drugs.pdf - Intelligently designed SWATH acquisition for novel psychoactive substances (NPS) detection in whole blood. SCIEX technical note RUO-MKT-02-9780-A.
- The Controlled Substance Act.(Title 21 United States Code (USC) Controlled Substances Act)
