Targeted and non-targeted analysis of fentanyl analogs and their potential metabolites using LC-QTOF
Abstract
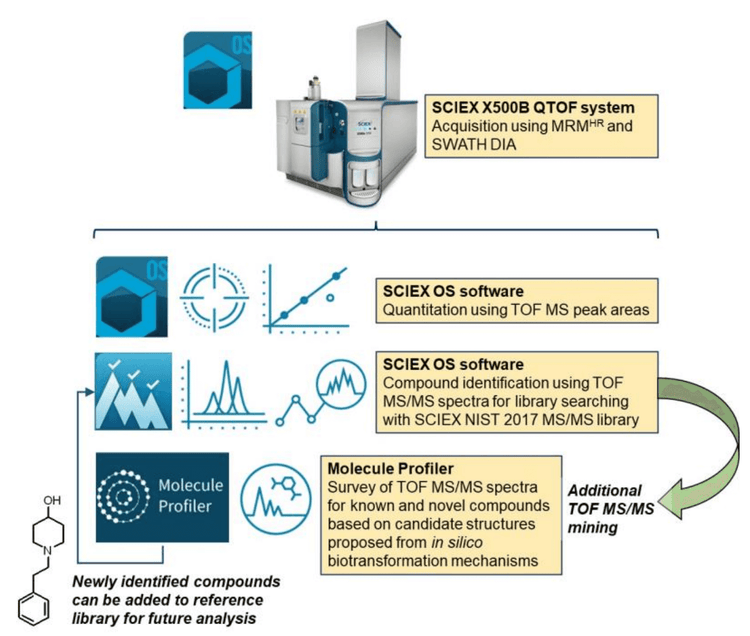
This technical note describes a high-throughput method that enabled the simultaneous quantitation and both targeted and non-targeted screening of fentanyl and its analogs in a single injection of urine samples. Using the X500B QTOF system, SWATH data-independent acquisition (DIA) collected MS/MS data for spectral matching against the SCIEX NIST 2017 MS/MS library and metabolite identification using the Molecule Profiler module of SCIEX OS software.
Introduction
The Centers for Disease Control and Prevention (CDC) estimates that approximately 150 overdose deaths occur daily due to synthetic opiates, such as fentanyl.1 The structural diversity of fentanyl complicates this public health crisis because as many as 1400 analogs are known to date.2 Forensic toxicology laboratories must perform immunoassay tests to screen for different drug classes in urine. However, their lack of specificity often requires LC-MS/MS analysis to quantify and identify specific compounds. This combined approach is lengthy and laborious and it often excludes new designer analogs that are illicitly produced. Under these less optimal synthesis and storage conditions, the resulting product might be an analytical cocktail of the known drugs and their analogs and impurities, all of which necessitate a non-targeted approach to capture as much information as possible for confident identification.
SWATH DIA enables the acquisition of high-resolution MS/MS spectra during each cycle, resulting in the collection of a MS/MS spectrum that is a composite of all the analytes within each isolation window. The resulting spectra can be retrospectively mined for further identification. Here, common MS/MS fragments of fentanyl and their structurally similar analogs were used to search for novel analogs and biomarkers of drug exposure in urine samples using the Molecule Profiler module (Figure 1).
Key features of SWATH DIA using the X500B QTOF system and SCIEX OS software
- High-throughput workflow combining quantitation based on TOF MS peak areas and both targeted and non-targeted screening based on TOF MS/MS spectra in a single injection
- SCIEX OS software offers a single platform for data acquisition, processing and rapid review of complex data
- Molecule Profiler module delivers an intuitive workflow for identifying fentanyl and its analogs in urine samples
Methods
Sample preparation: Frozen urine samples were thawed, vortex mixed, allowed to settle and then diluted 5-fold with 1:1 (v/v), methanol/water for LC-MS/MS analysis.
Standards for 27 fentanyl analogs, including fentanyl and nonfentanyl analog opiates, were purchased from Cerilliant. A solvent-based calibration curve was prepared in 1:1 (v/v), methanol/water, at concentrations ranging from 1 to 100 ng/mL.
Chromatography: LC separation was performed on an ExionLC system using a Phenomenex Luna Omega Polar C18 column (150 × 3.0 mm, 3.0 µm). A flow rate of 0.8 mL/min, an injection volume of 2 µL and a column temperature of 40°C were used. The 15-minute gradient used is shown in Table 1.
Mass spectrometry:The X500B QTOF system was used in positive electrospray ionization mode to acquire TOF MS and TOF MS/MS data with SWATH DIA. Table 2 shows the method parameters used for the mass spectrometer. The SWATH DIA method consisted of 8 windows, each 35 Da wide, over a start and stop precursor mass range of 200 to 450 Da.
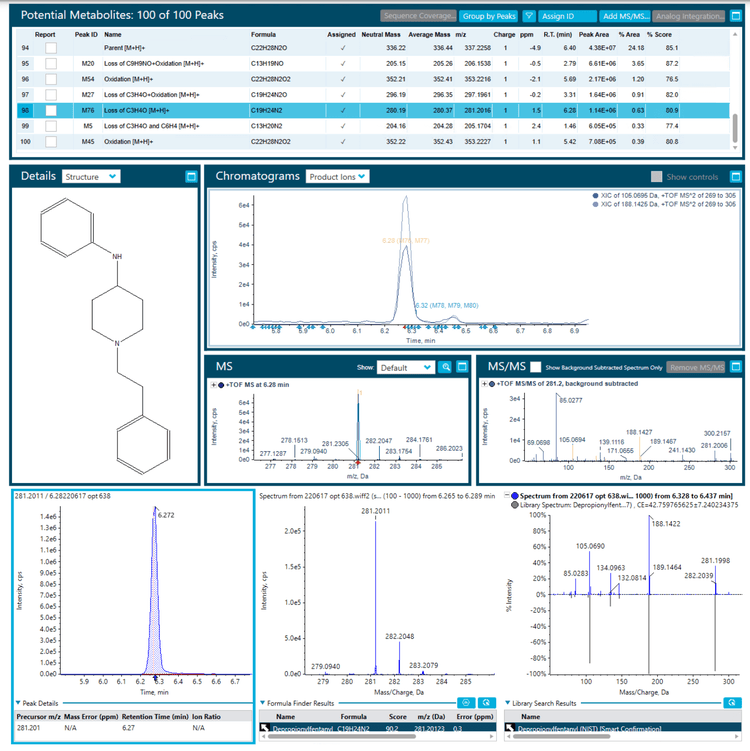
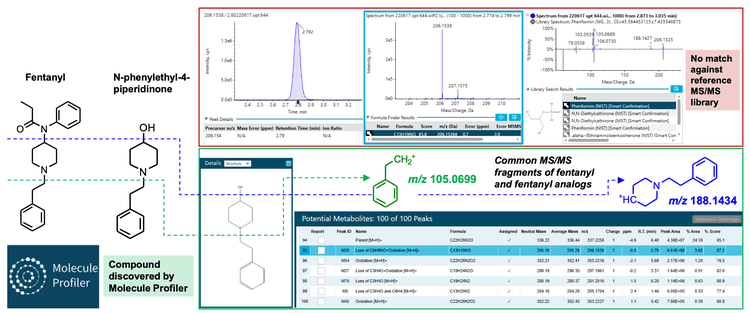
Data processing: All data were acquired and processed using SCIEX OS software, version 2.2. The SCIEX NIST 2017 MS/MS library was used to perform library searching in the Analytics module of SCIEX OS software. Screening for drug impurities, fentanyl analogs and candidate metabolites was performed using the Molecule Profiler module of SCIEX OS software. Candidate precursors were surveyed in the TOF MS/MS data based on the common fragments at m/z 188.1434 and 105.0699 for fentanyl and fentanyl analogs. Figure 2 shows the overall workflow.
Preliminary analysis of fentanyl and analog composition in urine samples
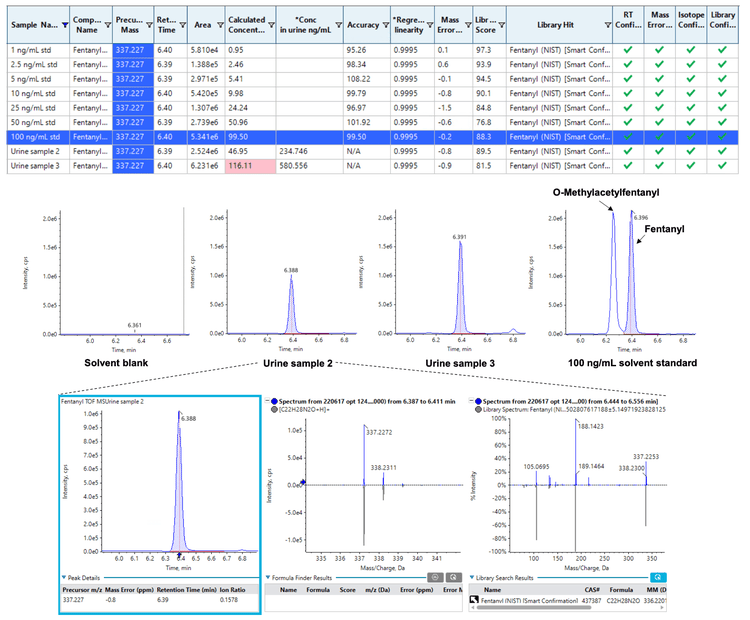
Urine samples were pre-screened to determine the composition of fentanyl, fentanyl analogs and other drug compounds using a solvent-based calibration to estimate their relative concentrations in each sample. Quantitation was performed based on the TOF MS peak areas. Fentanyl and acetyl norfentanyl were detected in most urine samples at approximately 8–4300 ng/mL and 26–470 ng/mL, respectively, while most other analytes were not observed. Figure 3 shows the results table containing the regression quality and concentrations of fentanyl in 2 urine samples, in addition to their extracted ion chromatograms (XICs) compared against a solvent blank and a standard. The calculated columns feature in SCIEX OS software was used to calculate the original concentrations in the urine samples based on the in-vial concentrations and 5-fold dilution from the diluteand-shoot protocol. Out-of-bound results were flagged, including, for example, the in-vial concentration of urine sample #3 that exceeded the upper limit of quantitation and would require dilution for re-analysis. The relative distribution of drugs and their estimated concentrations reported here informed the selection of representative urine samples for the subsequent analysis using library matching and Molecule Profiler.
Library matching
The TOF MS/MS data acquired for all urine samples were compared against the SCIEX NIST 2017 MS/MS library using the unknown screening workflow to confirm the known presence of fentanyl and its analogs from the pre-screening and to screen for other drug candidates, metabolites and impurities. By sorting on peak areas and library scores, a shortlist of candidates was used to build a new processing method using their known formula and the [M+H]+ adduct. The new processing method was then used to re-process the data with additional flagging for mass error and isotope ratio to bolster the confidence in any authentic findings.
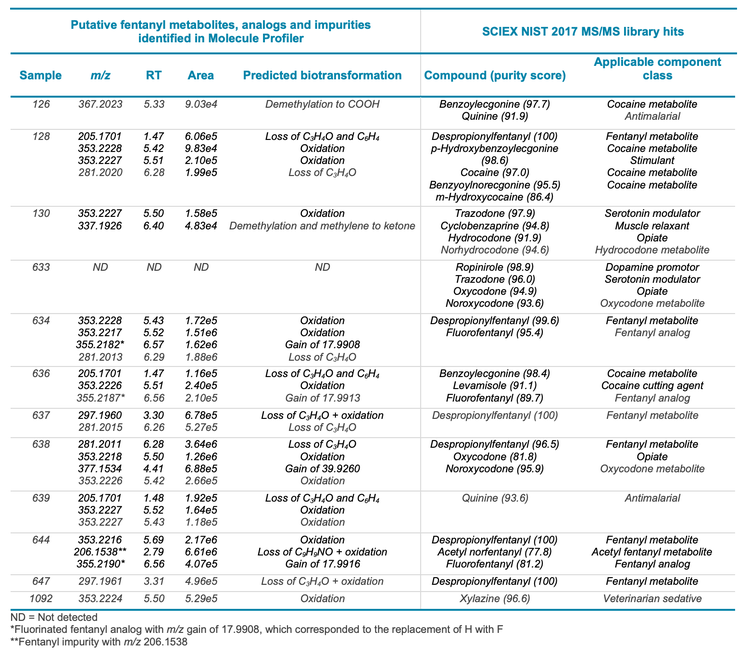
By defining confidence thresholds on retention time (RT) error, mass error, isotopic ratio matches and library hits, compound identification was rapidly achieved based on data that were flagged and filtered to pass pre-defined thresholds (Figure 3). Table 3 lists representative library findings that exhibited RT error of ≤5%, TOF MS mass error of ≤5 ppm, reasonable isotopic ratio matches and library scores of ≥75%. Most of the urine samples revealed the presence of other drug classes beyond fentanyl and fentanyl analogs, such as cocaine and opioids. Some of these samples were further interrogated with the Molecule Profiler module to corroborate the predicted compounds against the library results and identify additional biomarkers of drug use that were missed by library searching.
Detection of novel fentanyl analogs using Molecule Profiler module in SCIEX OS
The Molecule Profiler module of SCIEX OS software was used to search the dataset for potential precursors that shared the common fentanyl fragments, m/z 105.0695 and m/z 188.1425 (Figure 4). This analysis assumed that additional, novel fentanyl compounds also shared these common fragments. As shown in Figure 5, the Molecule Profiler module produced a list of proposed candidates that included fentanyl, fentanyl analogs, metabolites and impurities based on in silico biotransformation mechanisms in the software. For example, structural prediction of the selected peak at m/z 281.2016 corresponded to despropionylfentanyl (or 4-aminophenyl-1-phenethylpiperidine (4-ANPP)), which is often present as an impurity in drugs containing fentanyl and other analogs because it is a synthetic precursor of fentanyl. The identity of this compound was further corroborated by the similar RT, isotopic distribution and library hit observed in the Analytics module of SCIEX OS software.
In 3 urine samples, the Molecule Profiler module found a fentanyl analog with an m/z gain of 17.99 compared to the parent fentanyl, which corresponded to the replacement of hydrogen with fluorine (Table 3). By examining the same precursor m/z and retention time in the Analytics module of SCIEX OS software, this compound was confirmed to be o-fluorofentanyl based on the isotopic distribution and library hit.
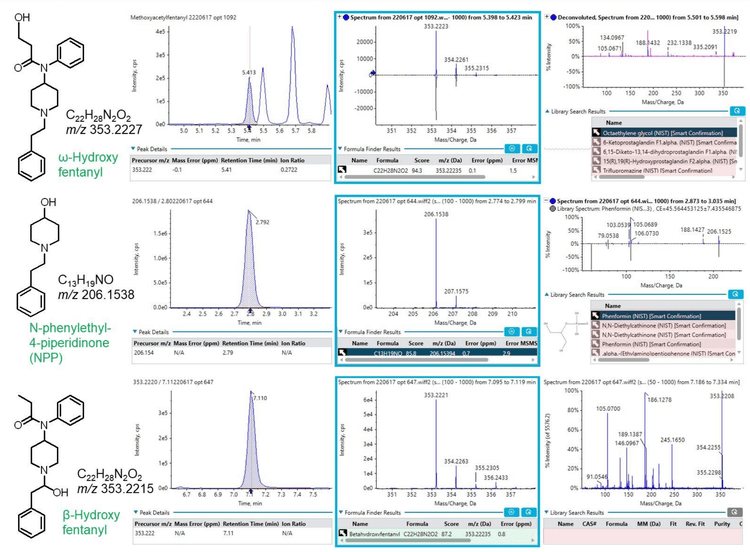
The Molecule Profiler module also identified several novel fentanyl biomarkers that were not found during library searching due to their absence from the SCIEX NIST 2017 MS/MS library (Figure 6). An oxidation product of fentanyl with m/z 353.2224 was consistently observed in many of the urine samples at 2 retention times (5.41 and 7.11 minutes). Using the structural *.mol file saved in Molecule Profiler to search on PubChem, these 2 biomarkers were identified as ω-hydroxy fentanyl and β hydroxyfentanyl. Both compounds were listed as “fentanyl-related substances with no known legitimate uses” in the International Narcotics Control Board (INCB). 3 β-hydroxyfentanyl is a fentanyl analog and was sold illicitly before being listed on the US Drug Enforcement Administration (DEA) National Forensic Laboratory Information System (NFLIS) drug substance list, 4 while ω-hydroxy fentanyl is a known metabolite of fentanyl. 5 Only β-hydroxyfentanyl was identified by a ChemSpider search on its molecular formula.
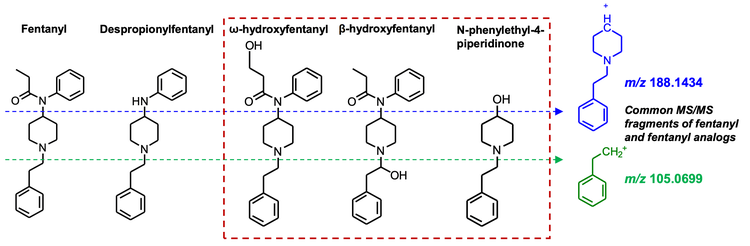
Another novel biomarker identified was N-phenylethyl-4- piperidinone (NPP) (Figure 6), a compound also listed in the NFLIS drug substance list.4 Like 4-ANPP, NPP can be used to synthesize fentanyl and thus might be present as a drug impurity.
Here, the Molecule Profiler module of SCIEX OS software provided an alternative way of processing TOF MS/MS data that enabled the discovery of novel compounds, which could be retroactively added to a reference library to improve future spectral matching, as shown in Figure 2.
Conclusion
- SWATH DIA of MS/MS spectra for all analytes during each cycle enables retrospective mining of previously acquired data for new substances that have emerged on the recreational drug market, without the need to re-acquire samples
- Simultaneous quantitation and library searching against >15,000 spectra from the SCIEX NIST 2017 MS/MS library
- Discovery of novel fentanyl biomarkers in the Molecule Profiler module of SCIEX OS software using common fragments of fentanyl and other analogs to screen for additional candidate precursors
- Integration of the Molecule Profiler software with SCIEX OS software enabled streamlined workflow for corroborating fentanyl analog and metabolite identification results against those found by targeted and non-targeted screening in the Analytics module of SCIEX OS software
References
- Ahmad, F.B., Cisewski, J.A., Rossen, L.M., Sutton, P. (2022) Provisional drug overdose death counts. National Center for Health Statistics.
- Misailidi, N., Papoutsis, I., Nikolaou, P., Dona, A., Spiliopoulou, C., Athanaselis, S. (2018) Fentanyls continue to replace heroin in the drug arena: the cases of ocfentanil and carfentanil. Forensic Toxicol. 36, 12-32.
- INCB. (2018). Fentanyl-Related Substances with No Known Legitimate Uses. https://www.incb.org/documents/Global_Projects_OPIOIDS/ 2018_June_24_INCB.OPIOIDS.Fentanyl_without_known_u ses.pdf: International Narcotics Control Board.
- US Drug Enforcement Administration, Diversion Control Division. (2021). 2017-2021 NFLIS-Drug Substance List. Springfield, VA: US Drug Enforcement Administration.
- Higashikawa, Y., Suzuki, S. Studies on 1-(2-Phenethyl)-4- (N-Propionylanilino)Piperidine (Fentanyl) and Its Related Compounds: Novel Metabolites in Rat Urine Following Injection of α-Methylfentanyl, One of the Most Abused Typical Designer Drugs. J. Health Sci. 54, 629-637.