Abstract
Quantitation of small molecule therapeutics in biological matrices is critical during the drug development process. The triple quadrupole platform offers excellent sensitivity and quantitative performance and has been a crucial driver for most bioanalytical methods. However, accurate mass spectrometry has increasingly been adopted for quantitative bioanalysis.1,2 With the inherent advantage of greater selectivity, improved mass resolution and the flexibility of TOF MS/MS data analysis, the ZenoTOF 7600 system provides excellent quantitative performance in multiple dimensions.3
This technical note demonstrates a versatile and sensitive approach for small molecule quantitation using the ZenoTOF 7600 system. A lower limit of quantitation (LLOQ) of 0.025 ng/mL for pioglitazone and 0.25 ng/mL for tolbutamide was achieved with an linear dynamic range (LDR) of 5 and 4.6 orders of magnitude, respectively, using a Zeno MRMHR-based quantitation workflow.
Key benefits of using the ZenoTOF 7600 system for the quantitation workflows
- Offering a versatile quantitation experience: Flexibility to choose between different quantitation modes: Zeno MRMHR, MS1 and Zeno MS1-based workflows for sensitive and selective quantitation using a single MS method.
- Achieve sub-ng/mL level quantitation using the Zeno MRMHR method in minimally treated human plasma: Achieve a 0.025 ng/mL LLOQ for pioglitazone and a 0.5 ng/mL LLOQ for tolbutamide in human plasma using the ZenoTOF 7600 system with improved MS/MS sampling efficiency.=
- Ideal analytical performance: Achieve accurate quantitative performance across a LDR spanning 5 orders of magnitude.
- Streamlined data management: Data acquisition and processing are integrated into SCIEX OS software, a 21 CFR Part 11 compliance-ready platform.
Introduction
Triple quadrupole mass spectrometers are the workhorses of quantitative assays in the drug development pipeline. As the potent modalities emerge, the need for selectivity is being addressed using methods on high-resolution accurate mass spectrometers. Despite better-resolving power and providing higher mass accuracies, achieving the required sensitivity remains a challenge on accurate mass instruments, including time-of-flight (TOF) systems. The challenge is primarily due to the typical QTOF duty cycles of 5-25%. Consequently, this results in losing up to 95% of detectable ions with each scan in the MS region. To overcome the challenge of TOF systems, improvement in the duty cycle is needed to ensure sensitive and selective quantitation of drug candidates in the pharma pipeline.1,2
The ZenoTOF 7600 system is equipped with the Zeno trap, leading up to 20-fold sensitivity gain without compromising speed, resolution, mass accuracy or dynamic range. When activated, ions are accumulated in the Zeno trap before pulsed into the TOF. Accumulating ions in the trap increases ion transmission to the TOF accelerator, improving the duty cycle to ≥90% across the entire mass range while performing MS/MS.1,2 Unlocking higher sensitivity levels enables the detection of and quantitation of low-abundance compounds that would otherwise be undetectable. Zeno trap enables the ZenoTOF 7600 system to be highly advantageous for quantitative bioanalysis workflows.
This technical note shows different approaches for quantitation with signal-to-noise (S/N) comparison for different quantitation modes on the ZenoTOF 7600 system using small molecule analytes.
Methods
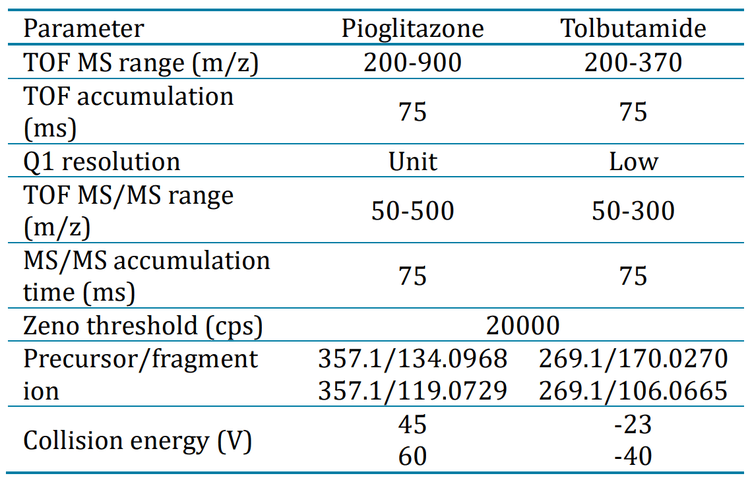
Sample preparation: Pioglitazone and tolbutamide stock solutions were prepared in dimethyl sulfoxide. Both analytes were spiked into 100 μL of human plasma at concentrations indicated in Figures 5A and 5B. A simple protein precipitation was performed with 200 μL of acetonitrile. Samples were vortexed for 30 seconds and centrifuged at 12000 rcf for 10 minutes at room temperature. The supernatant was transferred to an LC-MS vial for analysis.
Chromatography: Sample separation was performed using an ExionLC system at a 0.6 mL/min flow rate on a Phenomenex Kinetex XB-C18 (2.1 x 50 mm, 1.7 µm, 100 A ) column. A 3-minute gradient was run using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B (Table 1). The column temperature was maintained at 50°C. An injection volume of 1 μL was used for analysis. A mixture of 1 : 1 : 1 (v / v / v) acetonitrile:methanol : water was used as a needle wash solvent.
Exploring the different quantitation modes on the ZenoTOF 7600 system
Bioanalysis is imperative in characterizing critical factors in drug development, specifically for drug metabolism and pharmacokinetics (DMPK), which often requires targeted and low-level detection of pharmaceuticals in complex matrices. Both analytes achieved good sensitivity and selectivity using the Zeno MRMHR mode (Figures 2 and 3). Quantitation modes were specified in a single MS method file, acquiring data for all modes in a single run. Automated and accurate peak integration algorithms allowed rapid analysis through streamlined and integrated data processing.
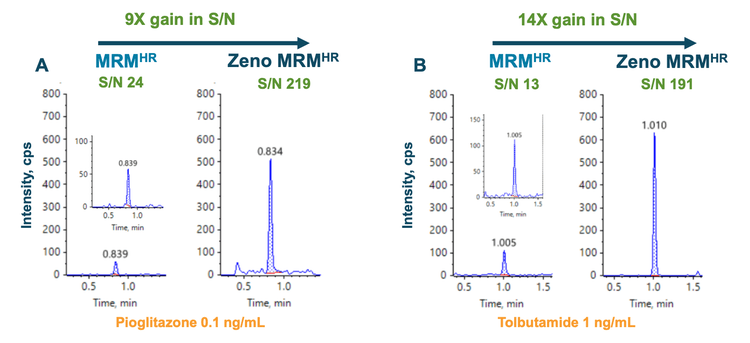
Zeno trap is a novel trapping/releasing technology integral to the ZenoTOF 7600 system and designed to overcome QTOF MS/MS duty cycle deficiencies. Figure 1 illustrates the Zeno trap in action in MRMHR mode. A 9-fold S/N gain was observed for pioglitazone (Figure 1A), and a 14-fold S/N gain was observed for tolbutamide (Figure 1B) compared to the conventional MRMHR experiment.
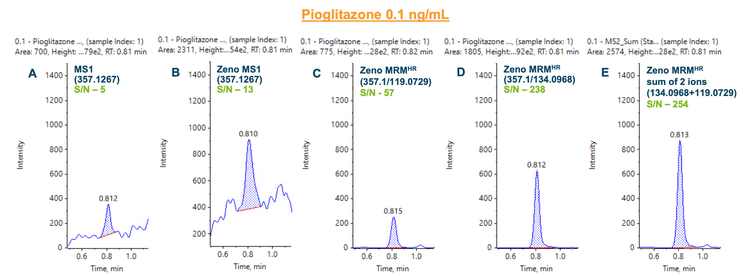
Figures 2 and 3 demonstrate the different flexible quantitation modes supported by the ZenoTOF 7600 system for pioglitazone and tolbutamide. MS1-based quantitation, which relies on precursor ions, offers a simple and easy-to-use method with reduced ion path tuning. With the Zeno trap enabled, a 3-fold improvement in the S/N was observed for pioglitazone based on MS1-level quantitation (Figure 2B). Using the Zeno MRMHR method, a S/N gain of 11-fold and 47-fold was achieved for the 2 most intense fragments, m/z 119.0729 (Figure 2C) and m/z 134.0968 (Figure 2D), respectively. Summation of the 2 most intense fragment ions resulted in a 51-fold improvement in S/N compared to MS1-based quantitation for pioglitazone.
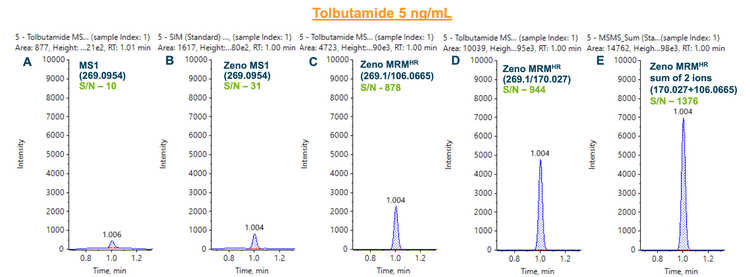
Figure 3 showed a similar outcome for tolbutamide. MS1- based quantitation was improved by 3-fold using the Zeno trap (Figure 3B). An 88-fold and 94-fold improvement in S/N was achieved using the Zeno MRMHR for the 2 most intense fragments, m/z 106.0665 (Figure 3C) and m/z 170.027 (Figure 3D), respectively. Summation of the 2 most intense fragment ions resulted in a 138-fold improvement in S/N compared to MS1-based quantitation.
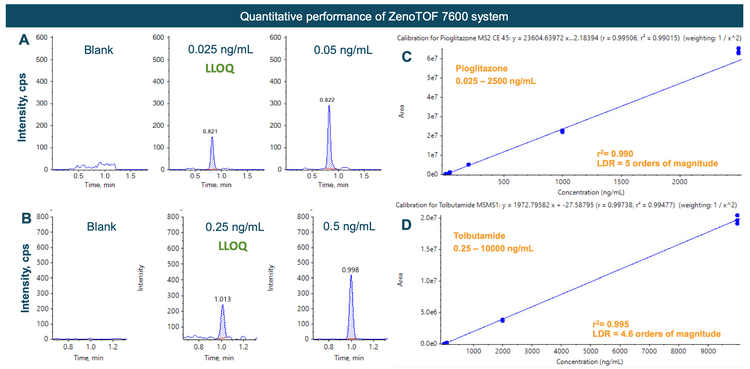
Quantitation performance using Zeno MRMHR
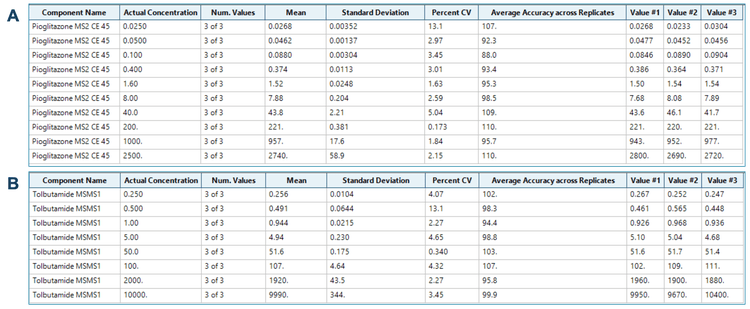
Linearity was achieved between 0.025 ng/mL and 2500 ng/mL with an r2 of 0.995 (Figure 4A) for pioglitazone and between 0.25 ng/mL and 10000 ng/mL with an r2 of 0.995 (Figure 4B) for tolbutamide. An LDR spanning 5 orders of magnitude was achieved for pioglitazone and 4.6 orders for tolbutamide.
Compliance-ready SCIEX OS software
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 6 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.

Conclusion
- A single MS method with various approaches for small molecule quantitation using Zeno TOF 7600 was employed and assessed to achieve the best sensitivity and selectivity for the quantitation of small molecules in a complex matrix
- A 9-fold and 14-fold gain in sensitivity (based on S/N) was achieved in the positive and negative modes using the Zeno MRMHR mode, respectively, compared to conventional MRMHR
- An LDR of up to 5 orders of magnitude was achieved for pioglitazone and 4.6 orders of magnitude for tolbutamide using Zeno MRMHR mode on positive and negative polarity
- A rapid analysis with minimal sample preparation was used to highlight the selective and sensitive quantitation of small molecules in human plasma
- SCIEX OS software is compliance-ready to support 21 CFR Part 11 and integrates with an accurate mass spectrometer to support data acquisition, processing and management on a single platform
References
- SCIEX Zeno trap whitepaper, RUO-MKT-19-13373-C
- Accomplish outstanding quantitative performance for bioanalysis of small molecule pharmaceuticals using accurate mass spectrometry. SCIEX technical note, RUO-MKT-02-15026-A

