LC-MS quantitation of retatrutide: triple receptor agonist for obesity and diabetes treatment
Abstract
This technical note demonstrates a sensitive method for the quantitation of retatrutide in human plasma using Zeno MRMHR on the ZenoTOF 7600 system. A lower limit of quantitation (LLOQ) of 2.5 ng/mL was achieved with simple and fast sample preparation (Figure 1).
The increasing prevalence of obesity and type 2 diabetes mellitus (T2DM) presents a major global health challenge, as many existing therapies lack adequate efficacy and safety.¹ As a triple-action agonist, retatrutide enhances satiety, suppresses hunger and boosts energy expenditure, potentially providing superior benefits for blood sugar control and weight loss compared to other incretin-based therapies.2 Based on preclinical data, retatrutide has shown a promising approach to addressing these unmet clinical needs. Therefore, when assessing levels of retatrutide in biological matrices for toxicokinetic and pharmacokinetic profiles, it is crucial to employ sensitive assays to help effectively evaluate the efficacy and the safety of the drug.
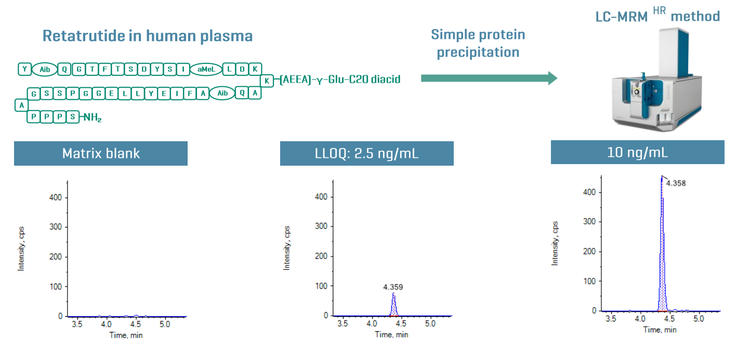
Key benefits for analysis of retatrutide using the ZenoTOF 7600 system
-
Sensitive quantitation of retatrutide: Achieve 2.5 ng/mL LLOQ for the quantitation of retatrutide in human plasma
-
Simple and fast sample preparation: Utilize a simple protein precipitation and achieve an average 89.6% recovery for the quantitation of retatrutide in human plasma
-
Robust analytical performance: Achieve highly reproducible quantitative performance with %CV <10 at all concentration levels across a linear dynamic range (LDR) of 3.6 orders of magnitude
-
Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplifies data acquisition and processing
Introduction
Retatrutide (LY3437943 or LY) is a 39-amino acid peptide engineered from a GIP backbone to act as a triple agonist at the glucagon (GCGR), GIP (GIPR), and GLP-1 (GLP-1R) receptors.³ The synergistic receptor activation enhances insulin secretion, improves glucose regulation and suppresses appetite, leading to better glycemic control and substantial weight loss. Clinical trials have shown significant efficacy, with marked reductions in body weight and improved glycemic outcomes, positioning retatrutide as a promising treatment for obesity and type 2 diabetes. Additionally, retatrutide has shown potential in lowering cardiovascular risk and treating non-alcoholic fatty liver disease, highlighting the broader impact on metabolic health.⁴
Due to its high efficacy in treating T2DM and obesity along with improvement of cardiovascular health, highly sensitive assays are necessary to ensure precise and accurate detection and quantitation when assessing the pharmacokinetic and pharmacodynamic effects.
Methods
Standard preparation: 1 mg of retatrutide standard was weighed and dissolved in 75:25 (v/v) methanol:water, with 0.1% formic acid as diluent.
Calibration curves (2.5–10,000 ng/mL) were prepared in human plasma by serial dilution and analyzed in triplicate. Pre- and post-spiked plasma samples (in 200 μL of human plasma) were prepared at 2.5, 7, 4000 and 8000 ng/mL, with each concentration analyzed in triplicate.
Sample preparation: Retatrutide was spiked into 200 μL of human plasma (2.5–10,000 ng/mL). Each sample was mixed with 600 μL of ice-cold methanol containing 0.1% formic acid, vortexed for 3 min at 2500 rpm, and centrifuged at 15,000 rpm for 5 min. A 100 μL aliquot of the supernatant was transferred to low-binding vials for analysis.
Chromatography: Analytical separation was conducted on an ExionLC 40 system with a Phenomenex Kinetex C8 column (2.1 × 100 mm, 2.6 μm) at 0.3 mL/min. Mobile phase A and B were 0.1% formic acid in water, and 0.1% formic acid in acetonitrile, respectively. The column was kept at 40 °C. Gradient conditions are summarized in Table 1. A 5 μL sample was injected for LC-MS analysis. Flow was diverted to waste from 0–3.5 min and 5–9 min to prevent contamination of the mass spectrometer.
Mass spectrometry: The optimized source and gas parameters are listed in Table 2 and the Zeno MRMHR parameters are included in Table 3.
Data processing: Analysis was performed using SCIEX OS software, version 3.4.5. Peaks were integrated using the MQ4 algorithm, and a weighting of 1/x2 was used for retratrutide quantitation. An XIC peak width of 0.02 Da was applied.
Quantitative performance on the SCIEX 7600 system
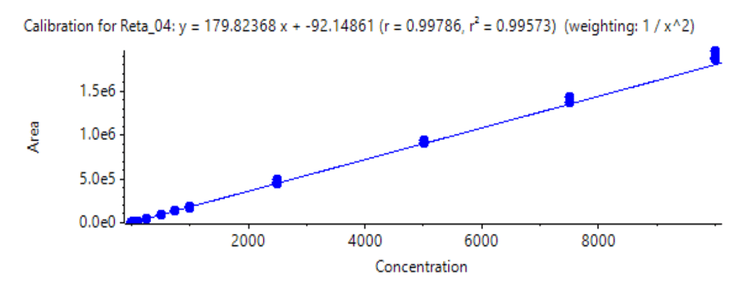
A triplicate injection was performed across concentrations ranging from 2.5 ng/mL to 10,000 ng/mL (Figure 2). An LDR of 3.6 orders of magnitude was achieved. A weighing factor of 1/x2 was used and a coefficient of determination (r2) of >0.996 was reached, showing excellent linearity across a wide calibration range.
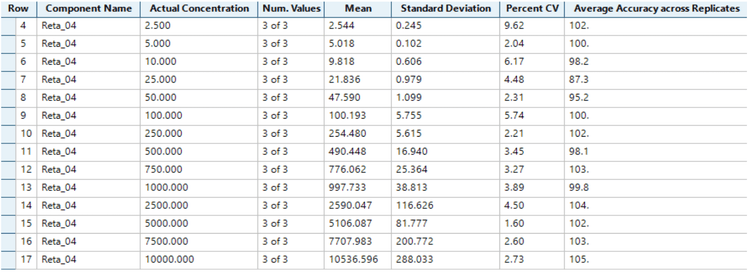
Analytical performance was evaluated based on the requirement that the accuracy of the calculated mean should be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean of the concentration should be below 20% at the LLOQ and below 15% at all higher concentrations.4
The assay accuracy was within ±13 % of the nominal concentration and the %CV was <10 for the quantitation of retatrutide in human plasma (Figure 3). Calculated %accuracy and %CV values were within the acceptance criteria at each concentration level.
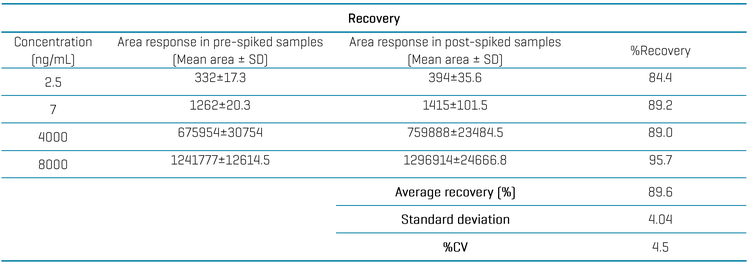
Recovery was evaluated for retatrutide at 4 different concentrations (2.5, 7, 4000 and 8000 ng/mL). A triplicate injection of the pre-spiked samples was compared to the post-spiked samples. The average recovery was 89.6% with a %CV of 4.5, achieved using a simple protein precipitation approach (Table 4).
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 7600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features. SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations.
Figure 4 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards.
The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.

Conclusions
-
An LOQ of 2.5 ng/mL was achieved for the quantitation of retatrutide in human plasma
-
Linearity was achieved at concentrations ranging from 2.5 ng/mL to 10,000 ng/mL with an r2 >0.996 covering an LDR of 3.6 orders of magnitude
-
Good quantitative performance was demonstrated with accurate and highly reproducible (%CV <10) results on the ZenoTOF 7600 system
-
An average recovery of 89.6% was achieved with %CV <4.5 using a simple sample preparation
-
Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support quantitative analysis on the ZenoTOF 7600 system
References
-
Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus, Kathleen Dungan, David M Nathan, Katya Rubinow, Feb 2025.
-
Peptide Sciences, Retatrutide, Dr. Hanna
-
LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept, Tamer Coskun et. al. August 2022
-
The power of three: Retatrutide's role in modern obesity and diabetes therapy. Volume 985, 15 December 2024, 177095 Toufik Abdul-Rahman et. al.
-
Bioanalytical Method Validation, May 2018
-
Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial, Ania M. Jastreboff et. al. Vol 389 no 6, June 26 2023


