Abstract
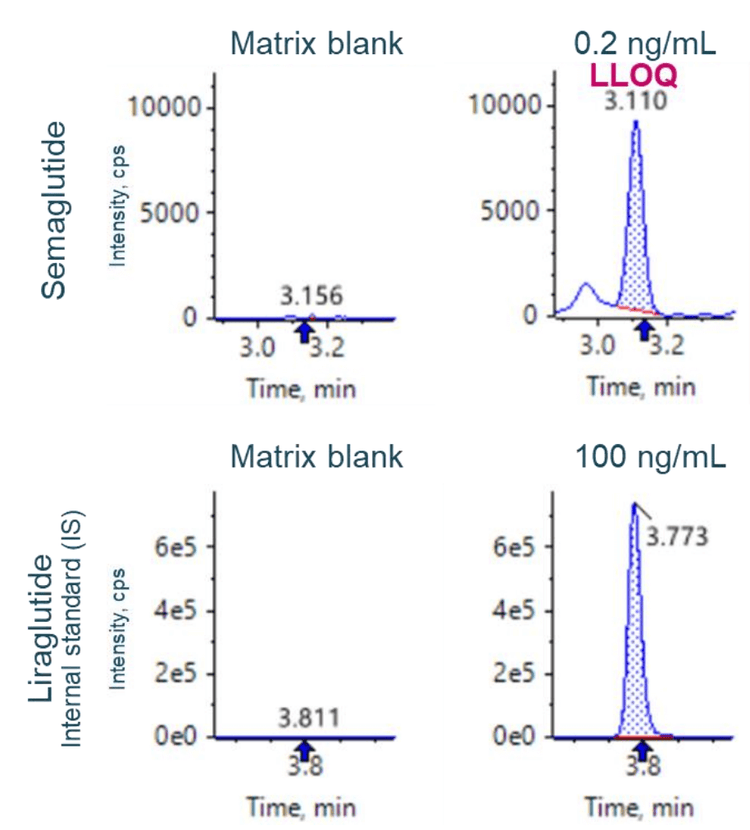
This technical note demonstrates a sensitive method to quantify a glucagon-like peptide-1 (GLP-1) analog, semaglutide, in rat plasma on a high-end triple quadrupole mass spectrometer. A lower limit of quantitation (LLOQ) of 0.2 ng/mL was determined using a 10-minute LC-MS/MS method (Figure 1).
Introduction
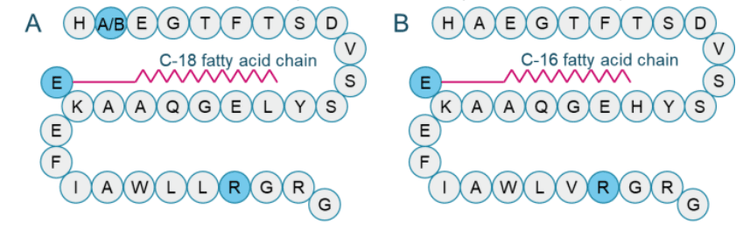
GLP-1 is an incretin hormone that stimulates glucose-dependent insulin release in humans. 1 GLP-1 analogs have attracted attention in drug development because they help regulate systemic homeostasis. Several GLP-1 analogs, including orally available semaglutide, are clinically approved and are used to treat type 2 diabetes. 1
Some GLP-1 analogs have low oral availability and are administered via subcutaneous injection in a frequent dosing regimen, leading to a treatment burden on patients. 2 When developing alternative formulations (non-injectables and longacting analogs), challenges arise, such as low bioavailability of the analogs in blood and limited sample availability. Such limitations impact the performance of quantitative assays used to assess pharmacodynamics throughout drug development. Therefore, sensitive and selective assays for high-confidence detection and quantitative performance in biological matrices are needed to ensure the safety and efficacy of the promising analogs.
The presented method demonstrates a sensitive quantitative method for a commercially available GLP-1 analog, semaglutide, in rat plasma. Sensitivity at the low-ng/mL level was achieved in rat plasma using the SCIEX 7500 system. Front-end enhancements on the instrument facilitated improved overall ion generation, capture and transmission, providing optimal quantitative sensitivity.
Key features of the quantitation of semaglutide using the SCIEX 7500 system
- Low-ng/mL level quantitation of a high-potency GLP-1 analog: Achieve a 0.2 ng/mL LLOQ for semaglutide in rat plasma on the SCIEX 7500 system
- Ideal analytical performance: Achieve accurate quantitative performance with %CV <13% at all concentration levels across a linear dynamic range (LDR) spanning 3 orders of magnitude
- Enhanced sensitivity unlocked: Improved front-end technology with the D Jet ion guide, OptiFlow Pro ion source and E Lens probe enhanced the ion generation, capture and transmission, enabling users to reach desired quantitative sensitivity3
- Streamlined data management: Data acquisition and processing are integrated into SCIEX OS software, a 21 CFR Part 11 compliance-ready platform
Methods
Sample preparation: The commercially available GLP-1 analogs, semaglutide (Figure 2A) and liraglutide (Figure 2B), were reconstituted in 6% formic acid in methanol and 3% formic acid in methanol, respectively. Semaglutide was spiked into 100 μL of rat plasma at concentrations ranging from 0.2 ng/mL to 200 ng/mL. Liraglutide was used as an internal standard (IS) and spiked at 10 ng. Protein precipitation was performed with 300 μL of methanol. Samples were vortexed for 30 seconds and centrifuged at 12000 rcf for 12 minutes at room temperature. The supernatant was transferred to a new Eppendorf tube. A 300 μL aliquot of 10% aqueous ammonia was added and gently mixed and 600 μL of the sample volume was transferred to an anion exchange Oasis MAX μ-Elution plate. Samples were washed with 5% ammonia in 1 : 1 (v/v) methanol/water. A second wash was performed using 1 : 2 : 2 (v/v/v) water : methanol : acetonitrile. Finally, elution was performed using 6% formic acid in 1 : 2 : 2 (v/v/v) water : methanol : acetonitrile mixture. The final elution volume was 100 μL.
Chromatography: Sample separation was performed using an ExionLC system at a flow rate of 0.4 mL/min on a Phenomenex Kinetex C18 (2.1 x 50 mm, 2.6 µm, 100 Å) column. A 10-minute gradient was run using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B (Table 1). The column temperature was maintained at 50°C. An injection volume of 5 μL was used for analysis. A mixture of 1 : 1 : 1 (v/v/v) acetonitrile : methanol : water was used as a needle wash solvent.
Quantitative performance
This technical note demonstrates a low-ng/mL level quantitation assay of semaglutide in rat plasma using the SCIEX 7500 system. Low solubility in an aqueous mixture and poor ionization of analogs are common challenges in the analytical detection of GLP-1 analogs. The method was optimized to achieve a sensitive quantitation assay from sample extraction to chromatography and MS detection.
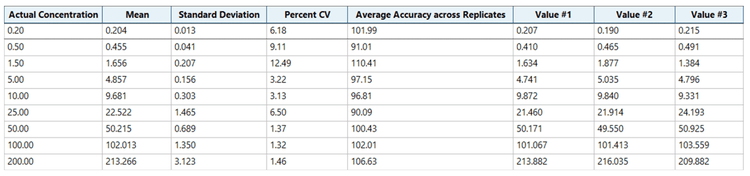
The calibration curve ranged from 0.2 ng/mL to 200 ng/mL and was prepared as described in the sample preparation section. Individual concentrations were run in triplicate
Analytical performance was evaluated for accuracy and precision. The accuracy of the calculated mean was expected to be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean for each concentration was expected to be <20% at the LLOQ and <15% at higher concentrations.
Accuracy was within ±11% of the nominal concentration and the %CV was <13% for semaglutide (Figure 3). Calculated accuracy and %CV values met the acceptance criteria at each concentration level.
Compliance-ready SCIEX OS software
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 5 illustrates the features of SCIEX OS software that are used for monitoring the audit trail, acquiring and processing data and configuring user access.

Conclusion
- An LLOQ of 0.2 ng/mL was reached for the quantitation of semaglutide in rat plasma
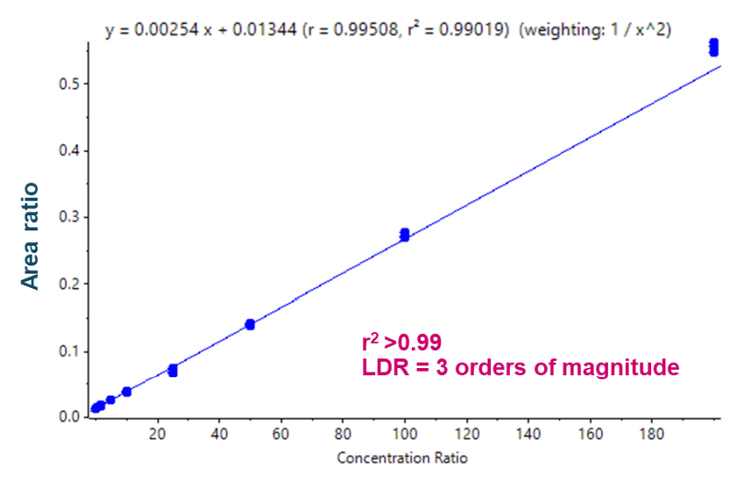
- Linearity was achieved between 0.2 ng/mL and 200 ng/mL, generating an LDR spanning 3 orders of magnitude with an r2 >0.99
- The method demonstrated accurate and highly reproducible (%CV <13%) quantitative performance at all concentrations
- Sensitivity was achieved on the SCIEX 7500 system with an improved front-end technology for better ion generation, capture and transmission
- SCIEX OS software is compliance-ready to support 21 CFR Part 11 and integrates with an accurate mass spectrometer to support data acquisition, processing and management on a single platform
References
- Wook Kim, Josephine M Egan (2008). The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512.
- Liana K Billings, Yehuda Handelsman, Michael Heile, Doron Schneider, Kathleen Wyne (2018). Health-Related Quality of Life Assessments with Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus. J Manag Care Spec Pharm. 2018 Sep;24(9-a Suppl):S30- S41.
- Enabling new levels of quantification. SCIEX technical note, RUO-MKT-02-11886-A.


