Abstract
Immunomodulatory drugs (IMiD) are a novel group of orally available chemotherapy agents. Pomalidomide is an IMiD that acts as a molecular glue and is administered in oral capsules alone or in combination with other drugs for treating multiple myeloma in humans. 1,2 Alternative formulations are needed for patients with dysphagia and pediatric patients who cannot swallow the drug.2
Challenges, such as low bioavailability, arise when developing alternative formulations. Therefore, sensitive and selective assays for high-confidence detection and quantitative performance in biological matrices are needed to ensure the safety and efficacy of promising IMiDs.
The presented method demonstrates a sensitive quantitation assay for pomalidomide using 25 μL of human plasma and a rapid protein precipitation method (Figure 1).
Key features of the quantitation of pomalidomide using the SCIEX 7500 system
- Sub-ng/mL level quantitation of an IMiD: Achieve a 0.1 ng/mL LLOQ for pomalidomide in human plasma using the SCIEX 7500 system
- Ideal analytical performance: Achieve accurate quantitative performance with %CV <10% at all concentration levels across a linear dynamic range (LDR) spanning 3.6 orders of magnitude
- Fast analysis with negligible carryover: Perform rapid LC-MS/MS analysis of pomalidomide in matrix with little to no carryover
- Enhanced sensitivity unlocked: Improved front-end technology with the D Jet ion guide, OptiFlow Pro ion source and E Lens probe enhanced the ion generation, capture and transmission, enabling users to reach desired quantitative sensitivity
- Streamlined data management: Data acquisition and processing are integrated into SCIEX OS software, a 21 CFR Part 11 compliance-ready platform
Introduction
Pomalidomide is one of the orally available IMiDs with potent antimyeloma activity used to treat patients who are exhausted from the frontline IMiD treatment or experiencing a relapse. 1 It exerts its potent antimyeloma and immune-stimulating effects by binding to its target E3 ligase, cereblon. Upon binding, endogenous proteasomal degradation is activated for the targeted removal of the transcription factors, Ikaros and Aiolos. 3
For patients that require alternative formulations, different drug combinations with pomalidomide and non-invasive routes of drug administration are tested to achieve improved clinical outcomes. With molecular glue degrader therapeutics on the rise, researchers are seeking analytical methods that can provide rapid quantitative answers to maintain the pace of drug development.
LC-MS/MS methods are increasingly applied for the quantitation of therapeutics as they offer the most sensitive and selective platforms for bioanalysis. Here, an LC-MS/MS quantitation assay for pomalidomide in human plasma is presented.
Methods
Samples and reagents: Pomalidomide was purchased from Tocris Biosciences and was reconstituted in dimethyl sulfoxide (DMSO).
Sample preparation: A working stock was prepared by diluting pomalidomide in acetonitrile. Individual concentrations were prepared using serial dilution of pomalidomide in acetonitrile. A 1.25 μL aliquot of the individual concentrations was spiked into 25 μL of human plasma to make a calibration range from 0.1 ng/mL to 400 ng/mL. Protein precipitation was performed with 75 μL of acetonitrile. Samples were vortexed for 30 seconds and centrifuged at 12000 rcf for 8 minutes at room temperature.
Finally, 50 μL of supernatant was transferred to injection vials for LC-MS/MS analysis.
Chromatography: Sample separation was performed using an ExionLC system at a 0.3 mL/min flow rate on an Acquity UPLC BEH C18 column (2.1 x 50 mm, 1.7 µm, 130 Å). A 5-minute gradient was run using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B (Table 1). The column temperature was maintained at 40°C and an injection volume of 10 μL was used for analysis. A mixture of 1:1:1 (v/v/v) acetonitrile:methanol : water was used as a needle wash solvent.
Mass spectrometry: The optimized source and gas parameters are listed in Table 2 and the optimized analyte-dependent MRM parameters are included in Table 3.
Quantitative performance
This technical note demonstrates a sub-ng/mL level quantitation assay of pomalidomide using 25 μL of human plasma on the SCIEX 7500 system. Low solubility and stability in an aqueous mixture are common analytical challenges experienced during the detection of pomalidomide. The method was optimized for a sensitive quantitation assay from sample extraction to chromatography and MS detection.
The calibration curve ranged from 0.1 ng/mL to 400 ng/mL and was prepared as described in the sample preparation section.
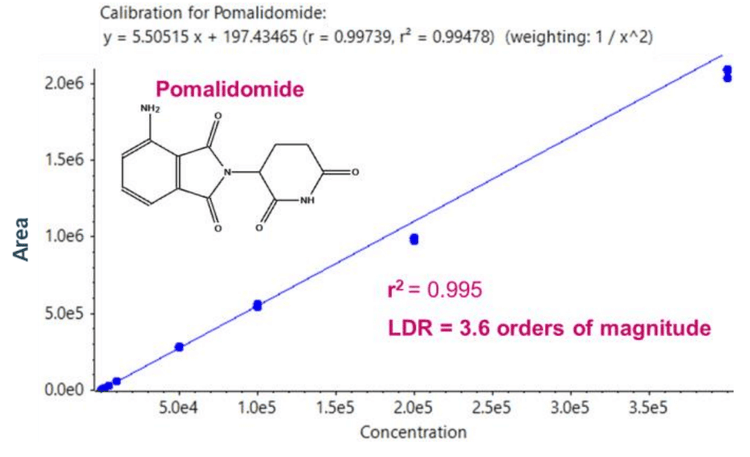
Individual concentrations were run in triplicate. Linearity was achieved between 0.1 ng/mL and 400 ng/mL with an r2 of 0.995 (Figure 2).
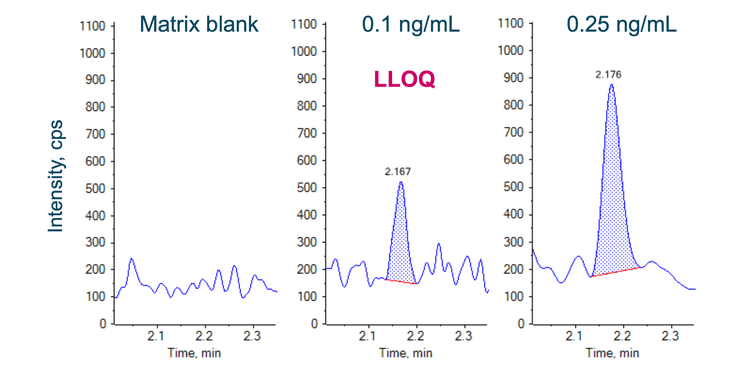
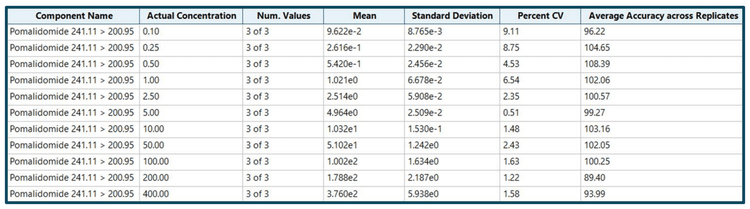
Analytical performance was evaluated for accuracy and precision. The accuracy of the calculated mean must be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean for each concentration must be <20% at the LLOQ and <15% at higher concentrations.
Accuracy was within ±11% of the nominal concentration and the %CV was <10% for pomalidomide (Figure 3). Calculated accuracy and %CV values met the acceptance criteria at each concentration level.
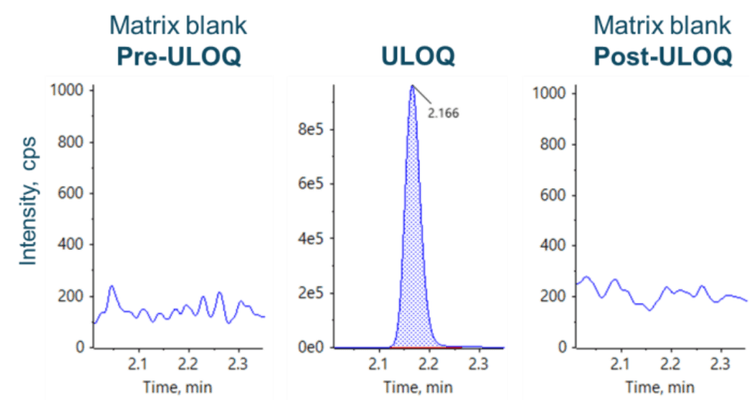
Carryover was assessed by injecting a matrix blank sample after the upper limit of quantitation (ULOQ) at 400 ng/mL. No visible peak was observed at the retention time of the analyte (2.176 min) in the post-ULOQ matrix blank (Figure 4).

Compliance-ready SCIEX OS software
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 5 illustrates the features of SCIEX OS software used for monitoring the audit trail, acquiring and processing data and configuring user access.
The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.
Conclusion
- An LLOQ of 0.1 ng/mL was reached for the quantitation of pomalidomide in human plasma
- Efficient 5-minute bioanalysis of pomalidomide was demonstrated using a rapid sample preparation, small sample volume (25 μL) and without visible carryover
- Linearity was achieved between 0.1 ng/mL and 400 ng/mL, generating an LDR spanning 3.6 orders of magnitude with an r 2 of 0.995
- The method demonstrated accurate and highly reproducible (%CV <10%) quantitative performance at all concentrations
- Sensitivity was achieved on the SCIEX 7500 system with an improved front-end technology for better ion generation, capture and transmission
- SCIEX OS software is compliance-ready to support 21 CFR Part 11 and integrates with a nominal mass spectrometer to support data acquisition, processing and management on a single platform
References
- Tomer Mark, Angelica Falkenstein, Jonathan Kish (2022). Real-world outcomes of pomalidomide therapy after lenalidomide induction in relapsed/refractory multiple myeloma. Future Oncol. 2022 Feb;18(5):553-564.
- Yan Li, Liangang Liu, Lian Huang, Xiaomin Wang, Matthew Hoffmann, Josephine Reyes, Maria Palmisano, Simon Zhou (2018). A Phase I, open-label, randomized, crossover study in healthy subjects to evaluate the bioavailability of, and the food effect on, a pomalidomide oral liquid suspension. Clin Pharmacol. 2018 Jul 19;10:89-99.
- David S Siegel, Gary J Schiller, Kevin W Song, Richy Agajanian, Keith Stockerl-Goldstein, Hakan Kaya, Michael Sebag, Christy Samaras, Ehsan Malek, Giampaolo Talamo, Christopher S Seet, Jorge Mouro, William E Pierceall, Faiza Zafar, Wiyuan Chung, Shankar Srinivasan, Amit Agarwal, Niza J Bahlis (2020). Pomalidomide plus low-dose dexamethasone in relapsed refractory multiple myeloma after lenalidomide treatment failure. British journal of haematology, 188(4), 501–510


