Abstract
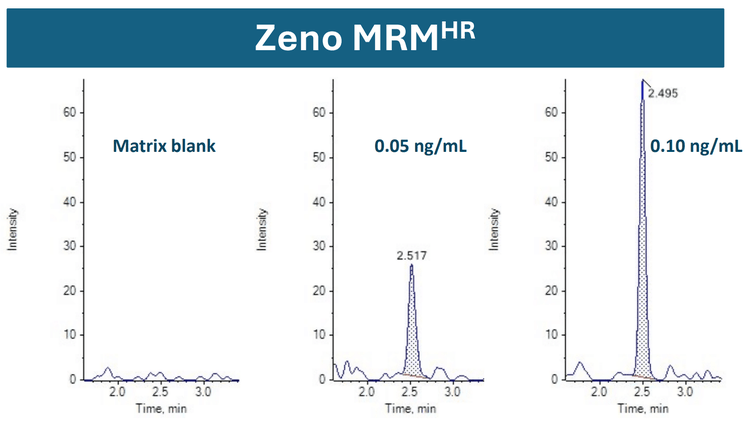
This technical note demonstrates a sensitive method for the quantitation of pasireotide in human plasma using high-resolution accurate mass spectrometry. A lower limit of quantitation (LLOQ) of 0.05 ng/mL was achieved in extracted plasma samples (Figure 1).
Pasireotide is a cyclic hexapeptide therapeutic introduced in 2012 for treating Cushing’s disease.1 Pasireotide is a pituitary-directed medication that helps reduce cortisol secretion from the adrenal glands. Due to its key role in treating Cushing’s disease, it is essential to effectively facilitate measurements of pasireotide at toxicokinetic and pharmacokinetic concentration levels in biological matrices.
This technical note demonstrates a reliable and highly sensitive workflow for supporting the quantitative analysis of pasireotide in human plasma using a high-resolution accurate mass spectrometer.
Key benefits for analysis of pasireotide using the ZenoTOF 7600 system
- Sub ng/mL level of quantitation: Achieve 0.050 ng/mL LLOQ for quantitation of pasireotide in human plasma
- Low plasma consumption: Reach low-level quantitation using 100 µL human plasma with increased MS/MS sampling efficiency using Zeno MRMHR
- Effortlessly meet critical quantitative performance criteria: Achieve accurate quantitative performance with %CV <11% at all concentration levels across a linear dynamic range (LDR) of 4.3 orders of magnitude
- Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplifies data acquisition and processing
Introduction
In 2012, Pasireotide was approved for treating adult Cushing's disease patients in both the EU and USA.2 It is specifically intended for those who are not eligible for surgery or have not seen improvement. Pasireotide was introduced as an alternative to somatostatin, the previous treatment option, which faced challenges due to its short half-life.3 The structure of pasireotide includes a disulfide bridge, which enhances its metabolic stability and makes it suitable for a prolonged pharmacological effect.4
As a result, the complex structures of cyclic peptide therapeutics such as pasireotide require highly selective and sensitive assays to ensure accurate detection and quantitation when evaluating pharmacokinetic and pharmacodynamic effects.
Methods
Standard preparation: One mg of pasireotide stock was procured from Medchem Express and dissolved in 0.1% formic acid in 50:50 (v/v) acetonitrile/water. Further, the dilutions were made in 0.1% formic acid in 20:80 (v/v) acetonitrile/water.
Sample preparation: Pasireotide (0.05 to 1000 ng/mL) was spiked into 100 µL of plasma. An equal volume of 50mM TRIS buffer was added to the plasma and vortexed. The samples were subjected to solid phase extraction using a Phenomenex Strata-X Polymeric Reverse Phase, 2mg 96 well plate. After loading, the sample was washed in water with 1% acetic acid in 95:5 (v/v) water/methanol. 5 Elution was performed with 200 µL of methanol and dried with low nitrogen. The samples were then reconstituted with 100 µL of 20:80 (v/v) 0.1% formic acid in acetonitrile/0.1% formic acid in water.
Chromatography: Analytical separation was performed on the ExionLC AE system using a Phenomenex Kinetex C18 (2.1 × 100 mm, 1.7 µm) column at a 0.3 mL/min flow rate. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. The column temperature was set to 55°C. The gradient conditions used are summarized in Table 1. A 20 µL sample aliquot was injected for LC-MS/MS analysis.
Quantitative performance on the ZenoTOF 7600 system
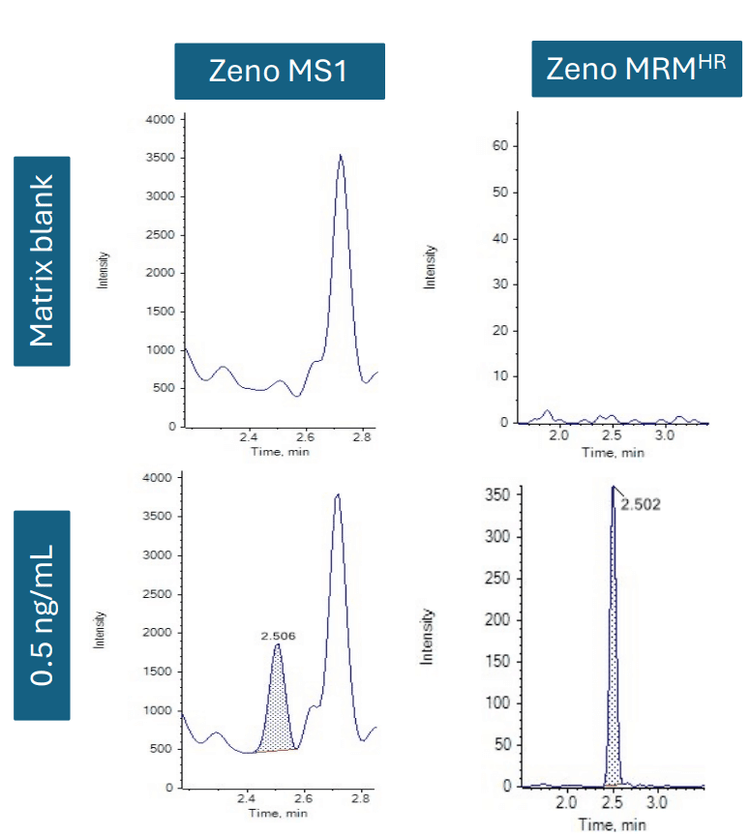
Cyclic peptide therapeutics such as pasireotide contain disulfide bridging, which can often introduce challenges with fragmentation. As an initial assessment, Zeno MS1 was evaluated, where precursor ion to precursor ion measurements were made without fragmentation. The approach was compared to Zeno MRMHR, where CID was applied and the product ion was measured. The matrix blank chromatograms were evaluated between Zeno MS1 and Zeno MRMHR modes. Compared to Zeno MRMHR, Zeno MS1 indicated lower selectivity with a higher background in the extracted plasma samples (Figure 2).
As a result, the Zeno MRMHR approach was used to achieve better selectivity and sensitivity for quantifying pasireotide in human plasma. The improved efficiency for total MS/MS sampling with the Zeno trap on the ZenoTOF 7600 system makes it a valuable tool for quantitative workflows that require high sensitivity and selectivity.
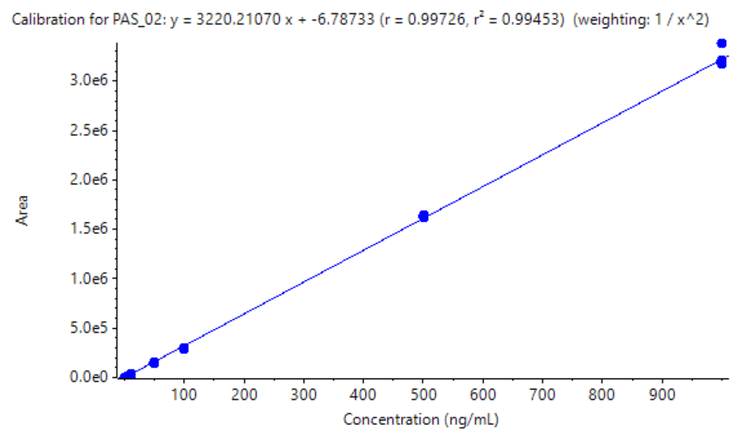
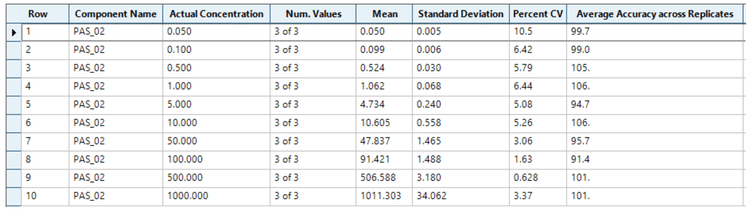
Using Zeno MRMHR, a calibration curve was analyzed for pasireotide at concentrations ranging from 0.05 to 1000 ng/mL. To evaluate reproducibility, each calibration standard was analyzed in triplicate. An LLOQ of 0.05 ng/mL was achieved with a minimal plasma volume of 100 µL (Figure 1). No matrix interference was observed at the retention time of the analyte.
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the SCIEX 7600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features.
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 5 illustrates the features of SCIEX OS software that are used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst, and reviewer.

Conclusions
- An LLOQ of 0.05 ng/mL was achieved to quantify pasireotide in human plasma
- Linearity was achieved at concentrations ranging from 0.05 ng/mL to 1000 ng/mL with an r2 >0.994 with minimal plasma volume
- Comparable quantitative performance was demonstrated with accurate and highly reproducible (%CV <11%) results on the ZenoTOF 7600 system
- A simple reverse phase solid phase extraction method was used to extract pasireotide from human plasma
- A single platform for streamlined data acquisition, processing, and management with SCIEX OS software was presented
- Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support regulated bioanalysis on the ZenoTOF 7600 system
References
- Treatment of Adrenocorticotropin-Dependent Cushing’s Syndrome: A Consensus Statement. The Journal of Clinical Endocrinology & Metabolism, 2008, 2454-2462.
- European Medicines Agency. Signifor solution for injection: summary of product characteristics. 2012.
- Pasireotide (SOM230): Development, mechanism of action and potential applications, Molecular and Cellular Endocrinology, 2008,286,69-74.
- A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential, Journal of Medicinal Chemistry, 2003, 46,12, 2334-2344.
- Quantitative analysis of pasireotide (SOM230), a cyclic peptide, in monkey plasma using liquid chromatography in combination with tandem mass spectrometry, Journal of Chromatography B, 1008,242-249, 2016.
- Sensitive quantitation of insulin glargine and its metabolites in human plasma, SCIEX technical note MKT32026-A
- Bioanalytical Method Validation, May 2018


