Abstract
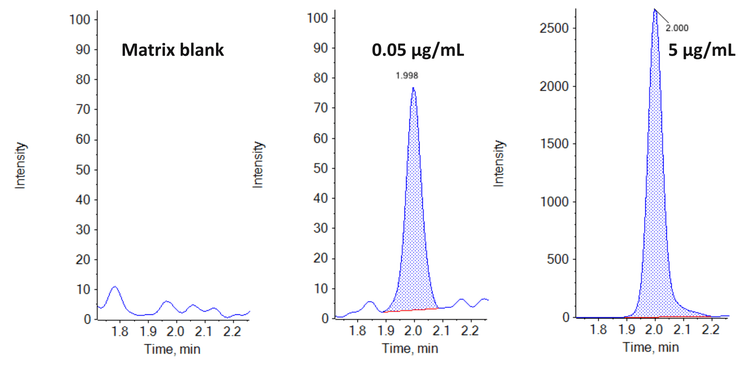
This technical note demonstrates a sensitive method for quantitation of trastuzumab deruxtecan (TDx) in human serum using high-resolution accurate mass spectrometry. A lower limit of quantitation (LLOQ) of 0.05 µg/mL (0.3 ng on column) was achieved in extracted human serum samples (Figure 1).
TDx was recently approved by FDA (2019) for treating breast, gastric and gastroesophageal where it functions by binding to HER2 of the malignant cells causing targeted DNA damage in cancer cells. It is an anti-HER2 ADC that comprises a HER2 monoclonal AB conjugate via internal cysteine residues.1 TDx has been known to express antitumor activity including in low HER2-expressing cancers.2, 3 As a result, it is critical to accurately measure levels of ADCs such as TDx in biological matrices for toxicokinetic and pharmacokinetic profiles to meet safety and efficacy requirements.
Key benefits for analysis of trastuzumab deruxtecan using the ZenoTOF 7600 system
- Sensitive quantitation of ADC: Achieve 0.05 µg/mL LLOQ for quantitation of TDx in human serum
- Low serum consumption: Low-level quantitation was achieved using 50 µL human serum with increased MS/MS sampling efficiency using Zeno MRMHR
- Effortlessly meet critical quantitative performance criteria: Achieve accurate quantitative performance with %CV <13% at all concentration levels across a linear dynamic range (LDR) of 3.7 orders of magnitude
- Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplifies data acquisition and processing
Introduction
ADCs have emerged as the most promising type of targeted cancer treatment with high target specificity.4 The structure of ADC comprises a monoclonal antibody (mAb) attached to a cytotoxic drug through a chemical linker. The mAb specifically targets the tumor antigen while the payload can be released to the site for its cytotoxic effects.5 Due to the specificity of ADCs towards cancer cells, such structures present high efficacy with minimal side effects. TDx is an anti-HER2 ADC with a specific target for tumor cells. Thus, it is very efficient for treating patients with breast and gastric cancers.
Due to its high potency in treating cancers, highly sensitive assays are necessary to ensure precise and accurate detection and quantitation when assessing pharmacokinetic and pharmacodynamic effects.
Methods
Standard preparation: 1 mg of TDx stock was procured from Medchem Express and dissolved in water. The dilutions were made in PBS for further processing.
Sample preparation: Dilutions were performed in human serum to prepare spiked sample concentrations ranging from 0.05 µg/mL to 250 µg/mL. Sample preparation was performed using 50 µL of spiked human serum, 200 µL of protein A beads and 200 µL of PBS. Protein A beads were washed three times with PBS before use. Samples were gently shaken for 45 minutes at room temperature, followed by two PBS washes. The beads were resuspended in 150 µL of digestion buffer containing 150mM ammonium bicarbonate and 1mM calcium chloride before denaturation at 95°C for 10 minutes. After allowing samples to cool to room temperature, 10 µg of trypsin was added to each sample, followed by on-bead digestion for 2 hours at 50°C. Digestion was stopped by adding 3 µL of formic acid. Samples were separated from the beads and transferred to vials for LC-MS/MS analysis.
Chromatography: Analytical separation was performed on the ExionLC AE system using a Phenomenex Aries peptide XB C18 (2.1 × 100 mm, 2.6 μ.m) column at a 0.5 mL/min flow rate. Mobile phase A was 0.1% (v/v) formic acid in water and mobile phase B was 0.1% (v/v) formic acid in acetonitrile. The column temperature was set to 40°C. The gradient conditions used are summarized in Table 1. A 20 μL sample was used for LC-MS/MS analysis.
Quantitative performance on the ZenoTOF 7600 system
The Zeno MRMHR technique provides superior sensitivity and selectivity for measuring TDx in human serum. For quantitative workflows requiring high sensitivity, the Zeno trap on the ZenoTOF 7600 system enhances the effectiveness of total MS/MS sampling.
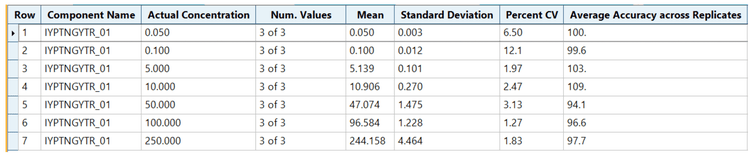
.The Zeno MRMHR method achieved excellent linearity, accuracy and precision from 0.05 µg/mL to 250 µg/mL for TDx. The on-bead digestion immunoprecipitation workflow resulted in clean samples that enabled a minimal human serum volume of 50 µL for the sensitive quantitation of TDx.
Linearity was achieved across concentrations ranging from 0.05 µg/mL to 250 µg/mL (Figure 2), achieving an LDR of 3.7 orders of magnitude.
Analytical performance was evaluated based on the requirement that the accuracy of the calculated mean should be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean of the concentration should be below 20% at the LLOQ and below 15% at all higher concentrations.4
This assay's accuracy was within ±15% of the nominal concentration and %CV was <13 for the quantitation of TDx in human serum (Figure 3). Calculated % accuracy and %CV values were within the acceptance criteria at each concentration level.
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 7600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features.
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 4 illustrates the features of SCIEX OS software that are used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst, and reviewer.

Conclusion
- An LLOQ of 0.05 µg/mL was achieved for the quantitation of TDx in human serum
- Low-level quantitation was achieved using 50 µL human serum given the increased MS/MS sampling efficiency with Zeno MRMHR
- Linearity was achieved at concentrations ranging from 0.05 µg/mL to 250 µg/mL, achieving an LDR of 3.7 orders of magnitude
- Comparable quantitative performance was demonstrated with accurate and highly reproducible (%CV <13%) results on the ZenoTOF 7600 system
- A single platform for streamlined data acquisition, processing, and management with SCIEX OS software was presented
- Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support regulated bioanalysis on the ZenoTOF 7600 system
References
- FDA approves fam-trastuzumab deruxtecan-nxki for HER2-positive gastric adenocarcinomas. Jan 2021
- Nagai Y, Oitate M, Shiozawa H, Ando O. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys, Animal Pharmacokinetics and metabolism. 49 (2019) 1086-1096. https://doi.org/10.1080/00498254.2018.1531158
- Toshihiko Doi, Hiroji Iwata, Junji Tsurutani, Shunji Takahashi, Haeseong Park, Charles H. Redfern, Kohei Shitara, Chikako Shimizu, Hiroya Taniguchi, Tsutomu Iwasa, Shinichiro Taira, Albert C. Lockhart, Jennifer M. Fisher, Takahiro Jikoh, Yoshihiko Fujisaki, Caleb C. Lee, Antoine Yver, and Kenji Tamura, Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study, The Lancet oncology. 18(11) (2017) 1512-1522. https://doi.org/10.1016/S1470-2045(17)30604-6
- Malik, P.; Phipps, C.; Edginton, A.; Blay, J. Pharmacokinetic considerations for antibody-drug conjugates against cancer. Pharm. Res. 34 (2017), 2579–2595. DOI: 10.1007/s11095-017-2259-3
- Nareshkumar Jain, Sean W. Smith, Sanjeevani Ghone Bruce Tomczuk, Current ADC linker Chemistry, 32 (2015) 3526-3540. https://doi.org/10.1007/s11095-015-1657-7
- Bioanalytical Method Validation, May 2018.



