Abstract
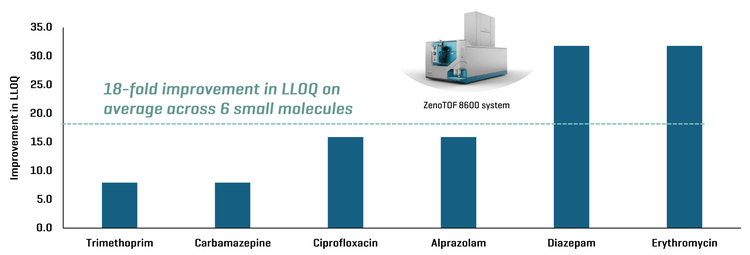
This technical note demonstrates a sensitive method for the quantitation of small molecule drugs in extracted rat plasma using the ZenoTOF 8600 system. On average, an 18-fold improvement in the lower limit of quantitation (LLOQ) was observed across 6 small molecules using the ZenoTOF 8600 system compared to the ZenoTOF 7600 system (Figure 1).
Due to their enhanced selectivity, which enables better LLOQs, the use of high-resolution mass spectrometry (HRMS) systems for drug discovery and development of small molecule therapeutics has grown. The quantitative sensitivity provided by these systems support critical workflows such as metabolite analysis, impurity analysis and bioanalysis to ensure proper safety and efficacy of small molecule therapeutics. To meet the need for improved selectivity and sensitivity, a novel quadrupole time-of-flight mass spectrometer—the ZenoTOF 8600 system – was used in this study to quantify small molecules in matrix. The overall duty cycle and hardware improvements in the DJet ion guide, QJet ion guide, OptiFlow Pro ion source and TOF optics enabled optimal sensitivity levels with excellent quantitative performance and wide dynamic range. Increased selectivity was also observed with matrix-challenged small molecules on the ZenoTOF 8600 system, enabling better sensitivity.
Key benefits for small molecule quantitation using the ZenoTOF 8600 system
- Improved LLOQ in matrix: An 18-fold improvement in LLOQ on average was demonstrated across 6 small molecules using Zeno MRMHR compared to the ZenoTOF 7600 system. Hardware improvements in the DJet ion guide, QJet ion guide, OptiFlow Pro ion source and TOF optics provided optimal quantitative sensitivity on the ZenoTOF 8600 system.
- Enhanced selectivity with HRMS: Low-level quantitation was reached for challenging small molecules when compared to traditional MRM analysis on a triple quadrupole mass spectrometer.
- Critical quantitative performance criteria met with ease: Accurate quantitative performance was achieved with %CV <13% at all concentration levels across a wide linear dynamic range (LDR) of up to 4.5 orders of magnitude.
- Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplified data acquisition and processing.
Introduction
HRMS systems are increasingly used to quantify challenging analytes, such as small molecules in biological matrices. The ZenoTOF 7600 system and ZenoTOF 8600 system offer greater sensitivity and enhanced selectivity, enabling users to perform trace-level analysis efficiently. In addition, HRMS systems allow for data collection over multiple fragment ions, which can be leveraged for better sensitivity for small molecule analysis.
As the need grows for greater selectivity and sensitivity for small molecule therapeutics, today’s HRMS systems have become an increasingly popular option. HRMS systems with greater MS/MS sampling efficiency, such as the previous generation ZenoTOF 7600 system, have enabled significant advancements in quantitative performance. In this study, the novel ZenoTOF 8600 system was applied for the quantitation of small molecules in rat plasma. The ZenoTOF 8600 system features enhanced hardware components—such as the DJet ion guide, QJet ion guide, OptiFlow Pro ion source and TOF optics—that enable better quantitative sensitivity and performance for pharmaceutical analysis.1
Methods
Sample preparation: Protein precipitation was performed using a ratio of 1:3 (v/v) rat plasma/methanol. Samples were vortexed and centrifuged at room temperature. The supernatant was further diluted at a ratio of 1:4 (v/v) supernatant/water. A mixture of 6 small molecules was spiked into diluted rat plasma extract and serially diluted to create a standard curve.
Chromatography: Sample separation was performed using a Phenomenex Gemini C18 column (3 x 50 mm, 3 µm, 110 Å) on an Exion AD system with a flow rate of 0.8 mL/min. For separation, a 4-minute method with 0.1% formic acid in water as mobile phase A and 0.1% formic acid in methanol as mobile phase B (Table 1) was used. The column was kept at 40°C. An injection volume of 5 μL was used for analysis. A mixture of 1:1:1 (v/v/v) acetonitrile/methanol/water was used as a needle wash solvent.
Mass spectrometry: Samples were analyzed using a ZenoTOF 8600 system operating in positive ion mode. The data were acquired using a Zeno MRMHR experiment. The optimized MS parameters are listed in Table2.
Data processing: A nalysis was performed using SCIEX OS software 3.5.0. Peaks were integrated using the MQ4 algorithm. An extracted ion chromatogram (XIC) peak width of 0.02 Da was applied for quantitation.
Quantitative performance with the ZenoTOF 8600 system
Typically, instrument performance is evaluated by comparing signal-to-noise (S/N) of a chromatographic peak. In this study, some of the small molecules analyzed in Zeno MRMHR showed very low background. Therefore, LLOQ was used as a metric to evaluate the 2 MS systems.
The quantitative performance of the ZenoTOF 8600 system was evaluated by analyzing 6 small molecules in the extracted rat plasma matrix. On average, an 18-fold improvement in LLOQ was achieved using the ZenoTOF 8600 system compared to the ZenoTOF 7600 system (Figure 1).
Figure 2 shows an XIC from representative small molecule examples that highlights the improvement in LLOQ with the ZenoTOF 8600 system compared to the ZenoTOF 7600 system. Alprazolam, diazepam and trimethoprim showed a 16-, 32- and 8-fold improvement in LLOQ, respectively, compared to the ZenoTOF 7600 system. Sensitivity enhancement is attributed to improvements in the source, ion guide and TOF optics, which enable low-level quantitation of small molecules in the matrix.
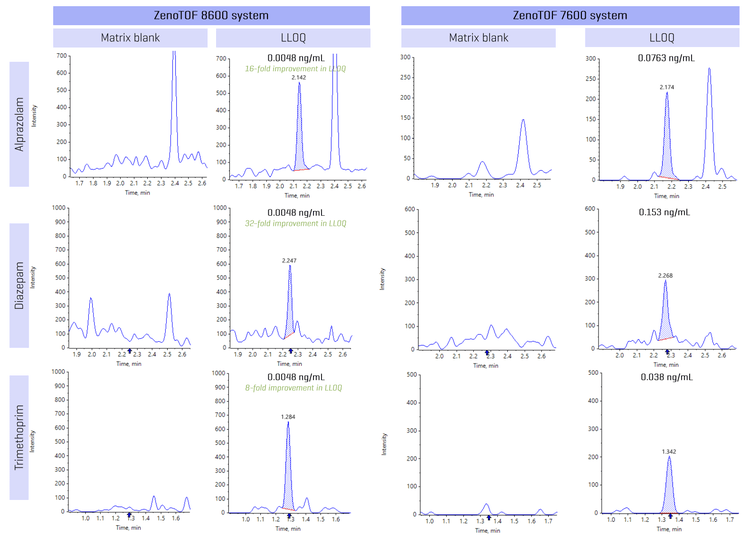
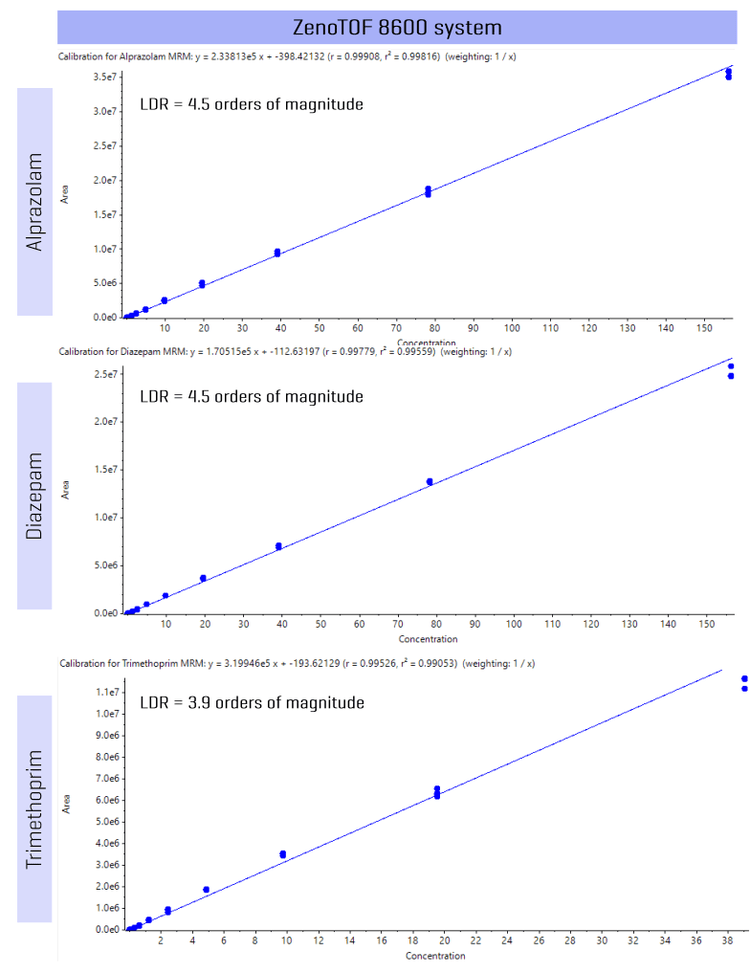
Figure 3 shows the calibration curve from the analysis of alprazolam, diazepam and trimethoprim where an LDR of up to 4.5 orders of magnitude was achieved, demonstrating that measurement across a wide range of concentration levels can be easily achieved on the ZenoTOF 8600 system.
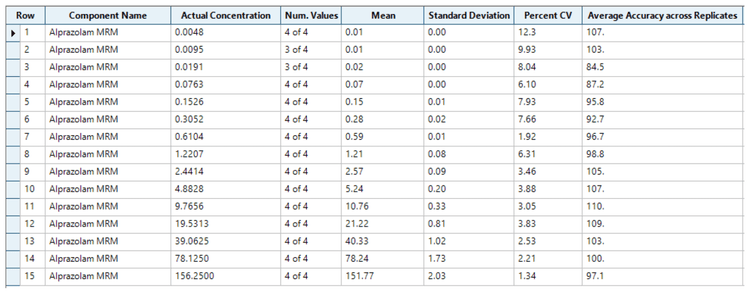
Evaluation of analytical performance was based on the requirement that the accuracy of the calculated mean should be 80%–120% at the LLOQ and 85%–115% at higher concentrations. The %CV of the calculated mean of the concentration should be <20% at the LLOQ and <15% at all higher concentrations.2
Selectivity enhancement for small molecule quantitation
Quantitative workflows are often faced with selectivity challenges, either high background or matrix interference. Since low-level quantitation is often necessary for drug discovery and development assays, high background and matrix interference can lead to a need for additional method optimization.
With HRMS systems, selectivity can be enhanced with greater mass resolution capabilities to differentiate the target analyte from matrix components. To evaluate selectivity on the ZenoTOF 8600 system, the same small molecules were analyzed on the SCIEX 7500+ system.
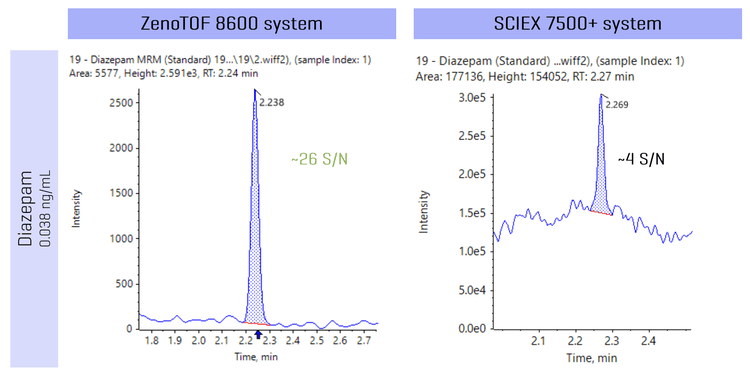
Figure 5 shows the analysis of a representative small molecule, diazepam, on the ZenoTOF 8600 system (Zeno MRMHR) and the SCIEX 7500+ system (MRM) at a concentration of 0.038 ng/mL. Analysis of diazepam showed a 6.5-fold improvement in S/N on the ZenoTOF 8600 system, demonstrating better selectivity when faced with high background ions. With the ZenoTOF 8600 system, the higher mass resolution allows for separation between target small molecules and background ions, allowing for better selectivity and quantitative sensitivity.
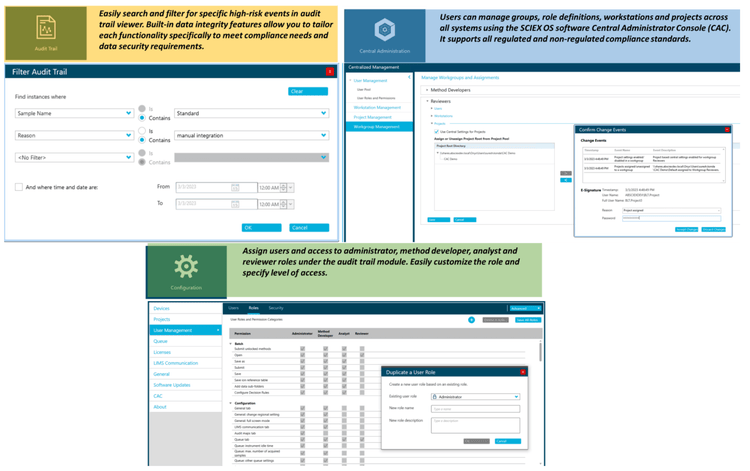
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 8600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features.
SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 7 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer.
Conclusions
- On average, an 18-fold improvement in LLOQ was reached for small molecule quantitation on the ZenoTOF 8600 system compared to the ZenoTOF 7600 system
- Enhanced sensitivity was achieved for small molecule analysis in matrix on the ZenoTOF 8600 system, given the improvements in hardware components such as the DJet ion guide, QJet ion guide, OptiFlow Pro ion source and TOF optics
- Improved S/N was observed for small molecules with challenging background ions on the ZenoTOF 8600 system when compared to traditional MRM analysis on a SCIEX 7500+ system
- Accurate quantitative performance was achieved with %CV <13% at all concentration levels across an LDR of up to 4.5 orders of magnitude
- Quantitative analysis was supported by data retention and compliance-readiness (21 CFR Part 11) features of SCIEX OS software on the ZenoTOF 8600 system
References
- XXX. SCIEX brochure, MKT-X-A.
- Enabling new levels of quantification. SCIEX technical note, RUO-MKT-02-11886-A.
- Enhanced sensitivity for peptide quantification in a complex matrix using high-resolution LC-MS/MS. SCIEX technical note, RUO-MKT-02-13324-A.
- US Department of Health and Human Services, Food and Drug Admiistration. Bioanalytical Method Validation: Guidance for Industry, May 2018.

