Abstract
An LC-MS/MS methodology for the rapid, quantitative analysis of uracil in human plasma and serum matrices has been developed. It demonstrates the sensitivity, linearity, and robustness of simple, isocratic chromatography coupled with automatable sample preparation. Preliminary results from investigations of uracil levels found in paired plasma and serum matrices from the same pool of research samples is shown.
Introduction
Researchers studying the toxicity of widely used fluoropyrimidine drugs, such as capecitabine and 5-fluorouracil, are interested in the analysis of the nucleobase uracil in biological matrices because, in theory, uracil levels may correspond to the degree of drug toxicity experienced by patients. Research has also suggested that fluoropyrimidine toxicity may be linked to genetic variance and research is ongoing to investigate whether uracil levels mirror this genetic stratification.
The analysis of very small molecules, including nucleobases such as uracil, presents unique challenges that must be overcome. Selectivity can be an issue, leading to poor sensitivity and the need for extensive sample preparation. LC-MS/MS, however, is one technique that can address these challenges. However, some molecules, particularly very small ones such as uracil, can present chromatographic and selectivity challenges for LC-MS/MS approaches, and appropriate methods need to be developed to fully leverage the potential of LC-MS/MS in terms of ease of use, simple sample preparation, sensitivity, linearity and reproducibility.
Key features of the SCIEX Triple Quad 6500+ system for the analysis of uracil
- High sensitivity was achieved, demonstrated by the 0.5 ng/mL limit of quantification (LLOQ) for uracil in a small volume of plasma/serum
- Strong quantitative performance was demonstrated by linear and reproducible results across all concentrations.
- Fast run times were achieved due to simplified chromatography, small sample volumes and straightforward extraction procedures.
Materials and Methods
Sample preparation: A 100 µL sample of plasma/serum was precipitated with acetonitrile:methanol, and the supernatant was diluted with water:formic acid. 25 µL of the supernatant was injected onto the LC-MS/MS system.
Chromatography: Chromatographic separation was achieved on a Phenomenex Kinetex PS C18 (2.6 µm, 4.6 x 100mm) column. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in methanol. The total run time was 5 minutes, at 97% mobile phase A. The flow rate was 0.45 mL/min.
Mass spectrometry: The SCIEX Triple Quad 6500+ system, operated with Analyst software 1.7.1, was used to analyze the samples. MRM parameters were optimized by infusion. TheIonDrive Turbo V ion source was operated in electrospray mode (ESI).
Data processing: A qualitative data review was performed using the Explorer module in SCIEX OS software. A quantitative analysis was performed using the Analytics module in SCIEX OS software.
Results
The LC-MS/MS methodology was developed using careful sample preparation and isocratic chromatography to maximize sensitivity, linearity and reproducibility.
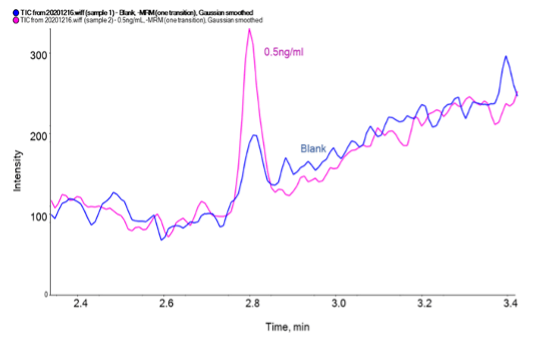
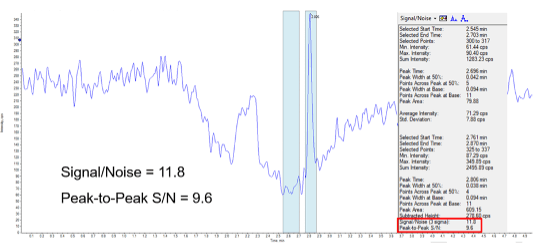
Sensitivity:The limit of quantification (LLOQ) for uracil in bovine serum albumin (BSA) was 0.5 ng/mL. Figure 2 shows the uracil peak at 2.806 min for a 0.5 ng/ml (LLOQ) in BSA sample. The blue bars provide a visual indication of the signal-to-noise ratio, which is greater than 10:1.
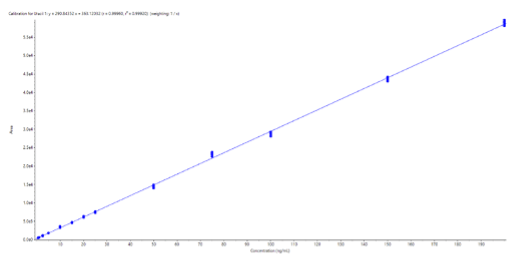
Linearity: Linearity was assessed over a concentration range of 0.5 to 200 ng/mL uracil in BSA, with 6 replicates at each concentration. Linear regression was used, based on peak areas and a 1/x weighting. The r2 value was greater than 0.999, indicating strong linearity in this concentration range. The resulting calibration curve is shown in Figure 3.
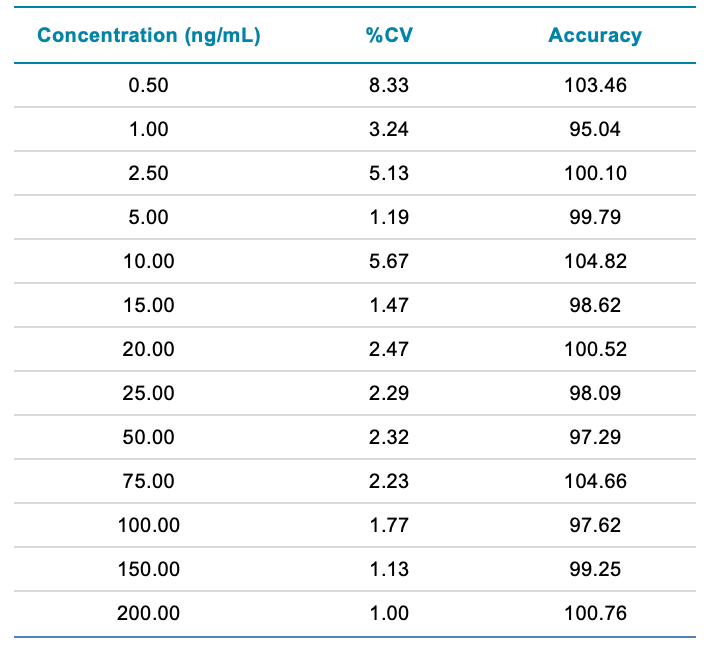
Reproducibility and robustness: To assess reproducibility and robustness, 6 replicates of calibration standards from 0.5 to 200 ng/mL uracil in BSA were analyzed. A summary of the results obtained is shown in Table 1. The results were highly reproducible, as the %CV was less than 8.5 at all concentrations tested.
Sample type comparison
Using the established and described method, a number of anonymized research samples were analyzed in both EDTA (plasma) and serum (Tables 2 and 3).
It was clear from the results that sample type was responsible for a significant difference in the uracil concentrations reported. For example, for sample number 24, the concentration of uracil in EDTA (plasma) was 23.54 ng/mL versus 8.66 ng/mL in the serum sample.
Conclusions
Methods were developed for the quantitative analysis of uracil in plasma and serum matrices using isocratic chromatography. A simple and automatable sample preparation method was developed using a protein precipitation from a 100 µL sample volume, with 25 µL injected onto the LC-MS/MS system. The LC-MS/MS methodology provided excellent sensitivity, linearity and reproducibility.
Preliminary results using LC-MS/MS show significant differences in uracil levels quantified from EDTA (plasma) and serum in the same research sample. Further studies on larger cohorts of samples are necessary to determine the best strategy or appropriate sample type for this analysis.
The preliminary data presented here show that the SCIEX Triple Quad 6500+ system is capable of quantifying uracil in biological matrices using a simple sample preparation, consisting of a protein precipitation from a small sample volume followed by robust and reproducible chromatography.
Acknowledgements
Plasma and serum samples used in this study were kindly provided by Viapath, UK.

