Abstract
During drug development, LC-MS/MS methods are required to deliver sufficient sensitivity and selectivity as well as high robustness such that large sample sets can be interrogated. High reproducibility is also required to meet bioanalytical requirements. Here, the performance of the SCIEX ExionLC 2.0 system on the SCIEX Triple Quad 5500+ system was investigated for linearity, precision and carryover for small molecule drug analysis.
Introduction
Robust LC-MS/MS methods are essential to support pharmaceutical drug development through all phases from discovery to clinical trials. The bioanalytical scientist is constantly challenged to achieve sufficient sensitivity and selectivity in method development, while maintaining robustness such that the final method can be used across large sample sets. At all concentration levels across the calibration range and from many different sources of matrix, the method must provide high reproducibility in order to produce consistent results.
An important aspect of a successful mass spectrometry (MS) experiment in achieving selectivity, reproducibility and robustness is combining it with some form of high-quality, up-front sample separation. In the majority of cases, this is achieved using liquid chromatography (LC). In this technical note, key performance of the SCIEX ExionLC 2.0 system was investigated, specifically linearity, precision and carryover, for small molecule drug analysis in rat plasma. A SCIEX Triple Quad 5500+ system was used for detection.
Key features of the ExionLC 2.0 system
- High-pressure, dual, serial piston pump rated to 860 bar at flow rates of 0.001 to 2 mL/min for maximum flexibility
- Precise and stable solvent flow delivering less than 1% RSD retention time variation
- Accurate and precise quantification results with linear coefficient of determination performance (r2) > 0.99 and precision <15% coefficient of variation for concentrations
Methods
Material and solutions: Assay linearity, precision and carryover were investigated using a SCIEX LC-MS Performance Essentials (SM Pharma) kit; part number 5056817.
Sample preparation: Calibration curves were prepared in precipitated rat plasma, and a bulk matrix sample was prepared using an acetonitrile precipitation approach. Three parts of cold acetonitrile were added to 1 part rat plasma. The solution was vortexed for 5 minutes, then centrifuged for 10 minutes at 10 000 x g. The supernatant was removed and added to an equivalent volume of water to make the final processed matrix solution. Standard solutions were prepared using a serial dilution approach using the prepared matrix solution. Table 1 shows the standards prepared.
Chromatography: LC separations were performed using the SCIEX ExionLC 2.0 system. A Phenomenex Kinetex C18 column (2.1 x 50 mm, 1.7 μm, P/N: 00B-4475-AN) was chosen, and a simple gradient of water and acetonitrile, both containing 0.1% formic acid, was used with a flow rate of 0.5 mL/min. Column oven temperature was set to 40 ̊C. The analytical run including equilibration was 3.5 minutes. The syringe speed was set to Normal and the speed factor to 1. The SCIEX ExionLC 2.0 system autosampler was used in the standard configuration consisting of a 250 µL syringe, 100 µL sample loop, 250 µL buffer tubing and 15 µL needle tubing. For all injections, the µL pick-up plus mode was selected in order to minimize the injection cycle time whilst optimizing sample consumption. An injection volume of 5 µL was used. The ExionLC 2.0 system autosampler was operated in advanced rinse mode using 2 mL 100% isopropanol with 0.1% formic acid for wash solvent and a wash sequence that included 2 valve washes.
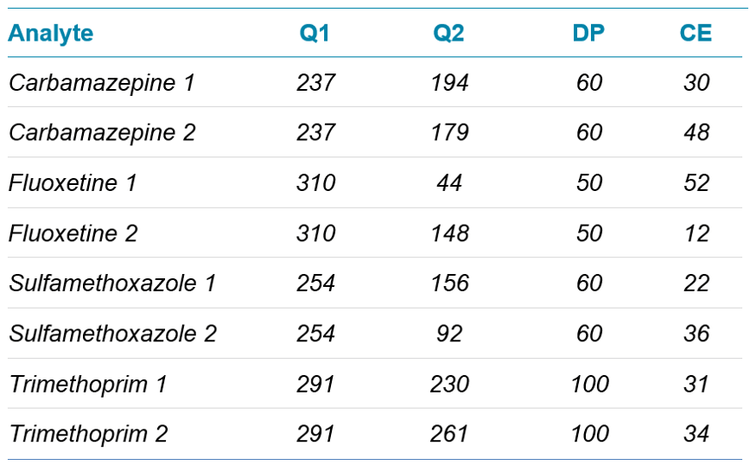
Mass spectrometry conditions: Mass spectrometry used a SCIEX 5500+ system. The ionization source was operated using electrospray ionization (ESI) in positive mode. Two MRM transitions were monitored for each analyte. The MRM conditions are detailed in Table 2.
Data acquisition was performed using Analyst software 1.7.1 with Components for the ExionLC 2.0 system. It is worth noting that the ExionLC 2.0 system is also fully supported for instruments in which data acquisition is performed using SCIEX OS software 2.1.5.
Data processing:Data processing of LC-MS data was performed using SCIEX OS software 2.0.1 in which calibration curves, precision and accuracy statistics were generated.
Results
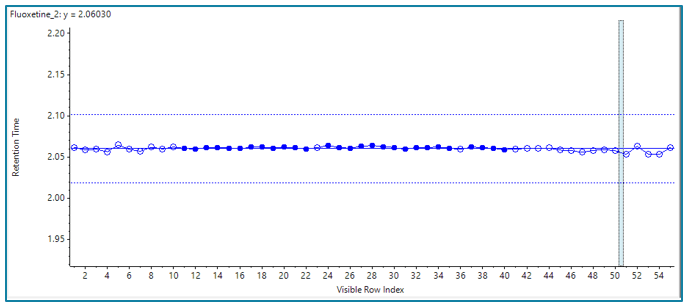
Figure 1 shows the retention time reproducibility for fluoxetine across 50 injections, highlighting the stability of the gradient generated by the ExionLC 2.0 system. Retention time precision of each of the analytes across a range of retention times for these injections is less than 1% RSD. Retention time variability %RSD for trimethoprim, sulfamethoxazole, carbamazepine and fluoxetine was 0.40, 0.10, 0.06 and 0.07 respectively, giving a maximum retention time difference of less than a second for all compounds.
An example chromatogram from std 8 (20 ng/mL) is shown in Figure 2 in which very good separation of the compounds was achieved.
The LLOQ of each analyte in matrix was determined based on current bioanalytical method guidance: the lowest standard concentration with a precision of <20%, accuracy between 80 and 120%, and a response (peak area) that is at least 5 times the response compared to the matrix blank.
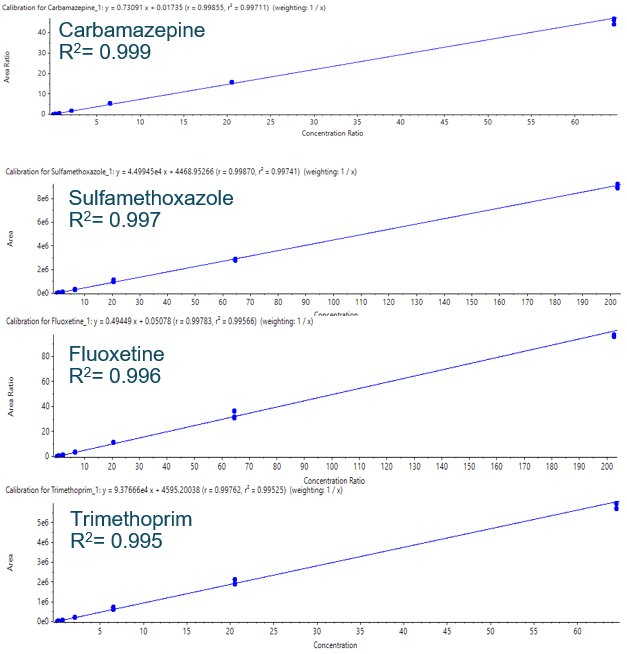
Listed in Table 3 are the LLOQs achieved for each analyte and the calibration curve range tested for each. Example calibration lines are shown in Figure 3. Linear regression with 1/x weighting was used. The linear coefficient of determination (r2) was higher than 0.99 for all compounds. The accuracy and precision of the calibration standards are shown in Table 4.
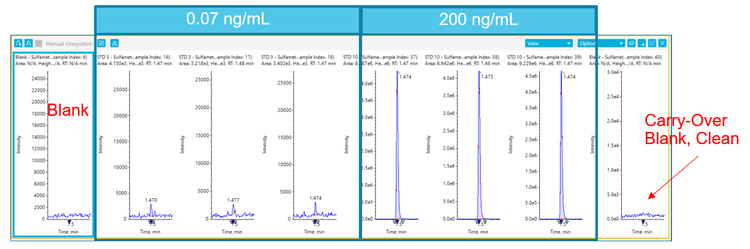
The concentration range tested in this work covers a typical linear dynamic range requirement when analyzing these small molecule pharmaceuticals in plasma. Figure 4 shows extracted ion chromatograms for sulfamethoxazole from extracted plasma standards and blanks. The blank injection after the standard 10 (200 ng/mL) shows no carryover peak detected showing that a simple and quick autosampler wash sequence provided acceptable carryover performance for the assay requirements.
Conclusions
The ExionLC 2.0 system has been shown to be a robust UHPLC system that is suitable for the bioanalysis of pharma small molecules in plasma matrix.
- Precise and stable solvent flow delivering less than 1% RSD retention time variation
- Accurate and precise quantification results with linear coefficient of determination performance (r2 > 0.99) and precision <15% coefficient of variation for the concentration range tested, typical for this small molecule pharmaceutical assay.




