Abstract
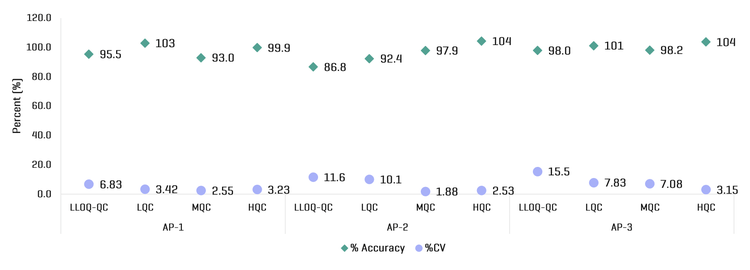
This technical note demonstrates sensitive small molecule quantitation using the ZenoTOF 7600 system. Using a Zeno MRMHR-based quantitation workflow, moxifloxacin in human plasma was quantified while maintaining consistent quantitative performance within- and between-runs (Fi gure 1).
Moxifloxacin is an antibiotic used to treat various bacterial infections. Fast and accurate analysis of antibiotic concentrations in complex matrices to monitor efficacy and toxicity is critical during treatment monitoring against bacterial infection. For years, triple quadrupole platforms have been the backbone of bioanalytical methods, providing exceptional sensitivity and quantitative performance for bioanalysis. However, accurate mass spectrometry has emerged as a powerful alternative to quantitative bioanalysis. Owing to the inherent advantage of greater selectivity, higher mass resolution and TOF MS/MS data analysis flexibility, the ZenoTOF 7600 system provides excellent quantitative performance with reliable bioanalytical output for small molecule analysis.1-3
Key benefits of using ZenoTOF 7600 system for quantitative workflows
- Maintain day-to-day quantitative performance: Accomplish consistent bioanalytical output within-run and between-runs with an overall accuracy within ±14% of the nominal concentration and %CV <16
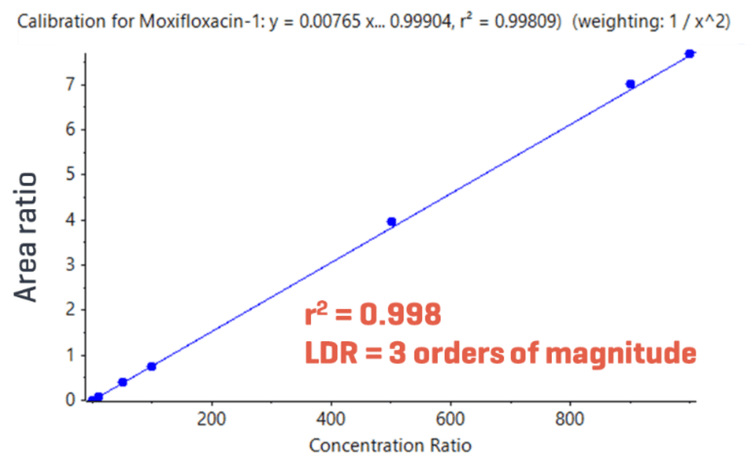
- Measure across a wide range of concentrations: Achieve highly accurate and reproducible quantitative performance across a wide linear dynamic range (LDR) spanning 3 orders of magnitude
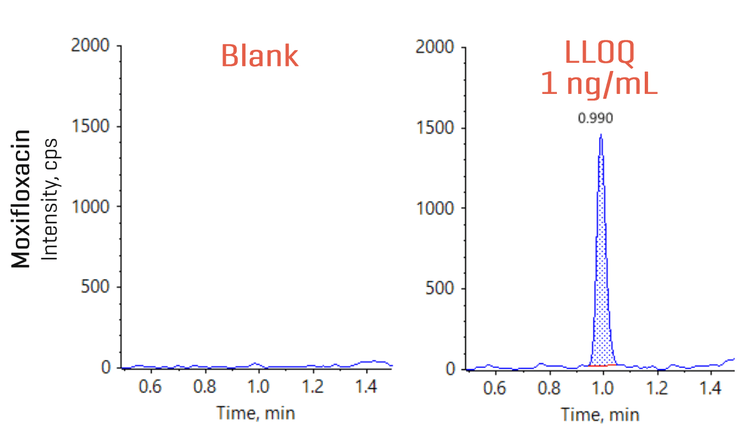
- Low-ng/mL level quantitation of moxifloxacin: Achieve 1 ng/mL LLOQ for moxifloxacin quantitation in human plasma
- Fast LC-HRMS analysis: Perform sensitive LC-MRMHR analysis of moxifloxacin in human plasma within a 5 min runtime
- Streamlined data management: Data acquisition and processing are integrated into SCIEX OS software, a 21 CFR Part 11 compliance-ready platform
Introduction
High-resolution mass spectrometry (HRMS) is increasingly recognized as an emerging platform for quantitative bioanalysis. While triple quadrupole systems remain the gold standard for targeted quantitation, HRMS platforms combine high mass accuracy, resolving power, and broad dynamic range to reliably quantify target analytes within complex biological matrices. At the same time, HRMS provides flexibility to identify co-eluting interferences, metabolites, and degradation products within a single analytical run.
Despite offering superior resolution and mass accuracy, achieving the required sensitivity for bioanalysis has traditionally been a challenge for HRMS systems, largely due to the inherent duty cycles of only 5–25%. To overcome this challenge, an improvement in the duty cycle is needed to ensure a sensitive and selective bioanalysis workflow. The ZenoTOF 7600 system addresses this need by integrating the Zeno trap technology, delivering up to a 20-fold gain in MS/MS sensitivity without compromising acquisition speed, resolution, mass accuracy, or dynamic range.¹,²
Applications such as therapeutic drug monitoring in tuberculosis (TB) research highlight the value of advanced HRMS platforms. Moxifloxacin, a key agent in treating multidrug-resistant TB, often exhibits low plasma concentrations and variable tissue distribution, necessitating highly sensitive and selective quantitation. The ZenoTOF 7600 system with SCIEX OS software (a 21 CFR Part 11 compliance-ready platform), enables precise quantitation of moxifloxacin in biological matrices, such as human plasma. The platform supports both research and regulated bioanalysis with greater confidence and efficiency while maintaining excellent day-to-day quantitative performance.
Methods
Sample preparation: 50 μL of spiked plasma samples, blanks and double blanks were prepared using serial dilution and transferred to a 96-well plate. Moxifloxacin-d4 was used as an internal standard and 25 μL was spiked in all wells, excluding double blanks. Protein precipitation was performed using 500 μL of acetonitrile. The plate was vortexed and centrifuged. 100 μL of supernatant from each well was transferred to a new plate and evaporated under nitrogen stream at 40°C. Dried samples were reconstituted using 100 μL 0.1% (v/v) formic acid in water for analysis.
Chromatography: Chromatographic separation was achieved using Waters XSelect CSH C18 column (2.1 mm x 50 mm, 2.5 μm, 130 Å). The ExionLC AD system was operated at a 0.5 mL/min flow rate. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. An injection volume of 20 μL was used for the analysis. The chromatographic gradient conditions are summarized in Table 1.
Quantitative performance
The ZenoTOF 7600 system, equipped with a Zeno trap, offers distinct advantages for accurate mass spectrometer-based bioanalysis. The Zeno trap allows more efficient ion transmission to the detector, enhancing MS/MS sampling and resulting in a sensitivity gain. Excellent linearity, accuracy, and precision were achieved using a sensitive and selective Zeno MRMHR-based quantitation workflow on the ZenoTOF 7600 system.
Figure 2 shows the XICs from blank and from the LLOQ level. An LLOQ of 1 ng/mL was achieved on the ZenoTOF 7600 system. No background interference was observed in the blank sample.
Compliance-ready SCIEX OS software
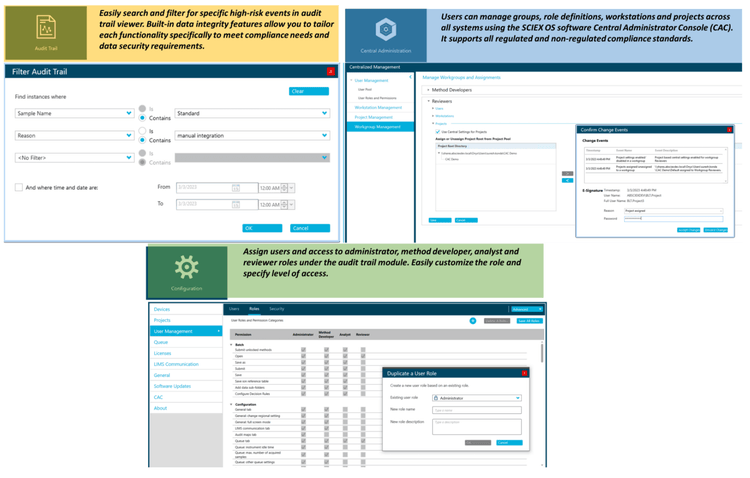
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 7600 system, ensuring high fidelity when performing method transfers while retaining critical compliance features. SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations. Figure 4 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity.
The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards. The configuration module allows users to assign roles and access as the administrator, method developer, analyst and reviewer
Conclusions
-
Day-to-day quantitative performance within-run and between-runs was maintained with an overall accuracy within ±14% of the nominal concentration and %CV <16
-
An accurate and highly reproducible assay was demonstrated across a wide LDR spanning 3 orders of magnitude
-
An LLOQ of 1 ng/mL was achieved for moxifloxacin using Zeno MRMHR mode on the ZenoTOF 7600 system
-
Fast and sensitive LC-MRMHR analysis of moxifloxacin in human plasma was performed within a 5 min runtime
-
Data management and compliance-readiness (21 CFR Part 11) features were highlighted on the SCIEX OS software, supporting non-regulated and regulated bioanalysis on the ZenoTOF 7600 system
References
-
SCIEX Zeno trap whitepaper, RUO-MKT-19-13373-C
-
Accomplish outstanding quantitative performance for bioanalysis of small molecule pharmaceuticals using accurate mass spectrometry. SCIEX technical note, RUO-MKT-02-15026-A
-
A versatile and sensitive approach for small molecule quantitation using an accurate mass spectrometer. SCIEX technical note, MKT-30560-A


