Abstract
LC-MS/MS is highly efficient for identification and quantitation of PPB levels of extractables/leachable compounds. The analytical method provides detection and quantification of the usual E&L found in the pharmaceutical containers and closures.
Introduction
The formulation and storage of drug products means that the product will come in contact with a variety of containers and packaging systems. Impurities from these packaging and transfer materials have the possibility of contaminating the drug product. Therefore it is very important to be able to measure whether any additional unwanted compounds have entered the drug product due to this contact. As per US FDA21CFR 211.94(a) “Drug product containers and closures shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug beyond the official or established requirements.”
The US FDA monitors levels of impurities due to container, closures, and packaging material contact. These impurities are categorized as extractables and leachables. Extractables are compounds that can be extracted from the container closure system when in the presence of a solvent, or other harsh condition, while leachables are compounds that leach into the drug product formulation from the container and/or closure as a result of direct contact with the formulation over time. The objective of extractables analysis involves identification and quantitation of impurities from the container, closures and packaging material, while for leachables the drug product is analyzed for contaminants. A sensitive and selective method for determination of a set of commonly monitored extractable and leachable compounds was developed and is described here.
The goal was to development a sensitive analytical method using the SCIEX Triple Quad™ 3500 System with an ExionLC™ AD LC System for simultaneous detection of 15 leachable and extractables commonly found in drug product, packaging, containers and closure.
Key features of LC-MRM for specific and sensitivity detection of E&L in drug products
- LC-MS/MS is an efficient platform for identification and quantitation of ppb levels of extractables and leachables
- The analytical method provides detection and quantification of the usual E&L found in the pharmaceutical containers and closures
- SCIEX TripleQuad 3500 System provides detection limits below regulatory requirements for these compounds.
Methods
Sample preparation: The standards of Antioxidant 425, Bbot 150, Diethylphthalate, Diphenylpthalate, Irgafos 168, Irganox 1010, Irganox 330, Irganox 565, Methylparaben, Propylparaben, Tinuvin 120, Tinuvin 328, Tinuvin p, Uvinul 3030, Uvinul 408 were prepared as a cocktail in 100% acetonitrile in a concentration range of 50 – 400 PPB.
For extraction of extractables and leachable from containers, 1g of the sample (Nasal spray container, Ophthalmic solution container) was weighed. Added 10 ml of n-Hexane solution and vortex. The samples were sonicated for 3hrs. The supernatant was collected and evaporated to dryness under nitrogen stream. The dried samples were reconstituted with Diluent (100% ACN).
For extraction of extractables and leachable from drug product, 4ml of sample was taken. Added 4 ml of Dichloromethane and vortex. The supernatant was collected and evaporated to dryness under nitrogen stream. The samples were then reconstituted in diluent (100% ACN).
Chromatography: Liquid chromatography separation was performed on the SCIEX ExionLC™ AD HPLC System at 30°C. Separation was achieved using a Phenomenex Kinetex column, with mobile phase 2mM ammonium formate in water with 0.1% formic acid and acetonitrile. Column temperature was 30 ºC and the injection volume was 10 µL. More details on the chromatography can be found in the Supplementary Information.4
Mass spectrometry: The SCIEX Triple Quad™ 3500 System was operated in positive electrospray ionization mode with unit resolution for both Q1 and Q3. The MS compound parameters (DP, EP, CE, CXP) were optimized individually for each analyte (Supplementary Information).4
Data processing:The software program Analyst® Software 1.6.3 was used for instrument control, data acquisition and processing. A 1/x2 weighted linear regression was used to calculate the concentrations.
Results
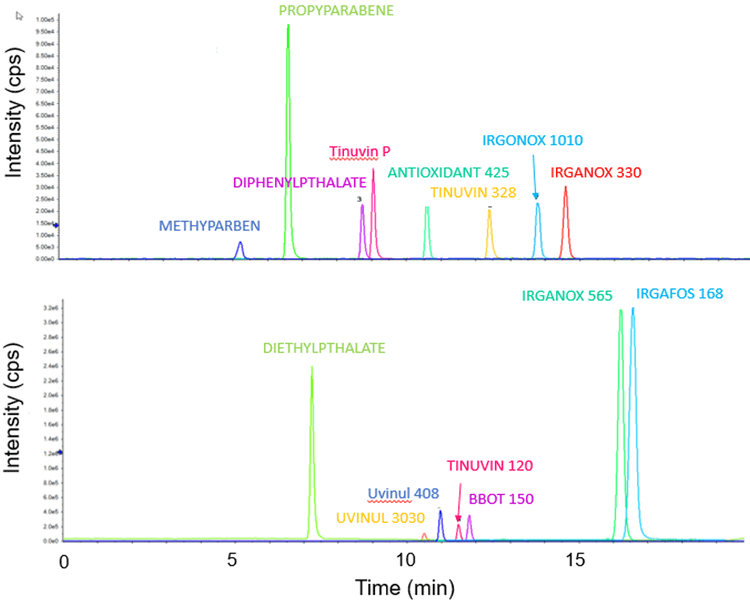
Good separation was achieved using the Kinetix column, the chromatogram (XIC) for all 15 analytes analyzed by using LC-MS/MS is shown in the Figure 2. The expected retention time when using this method is provided in the method information in the Supplementary information.4
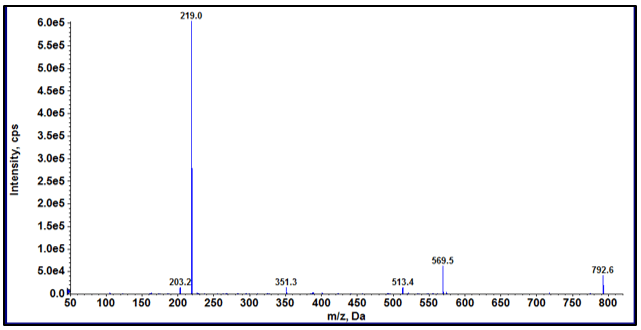
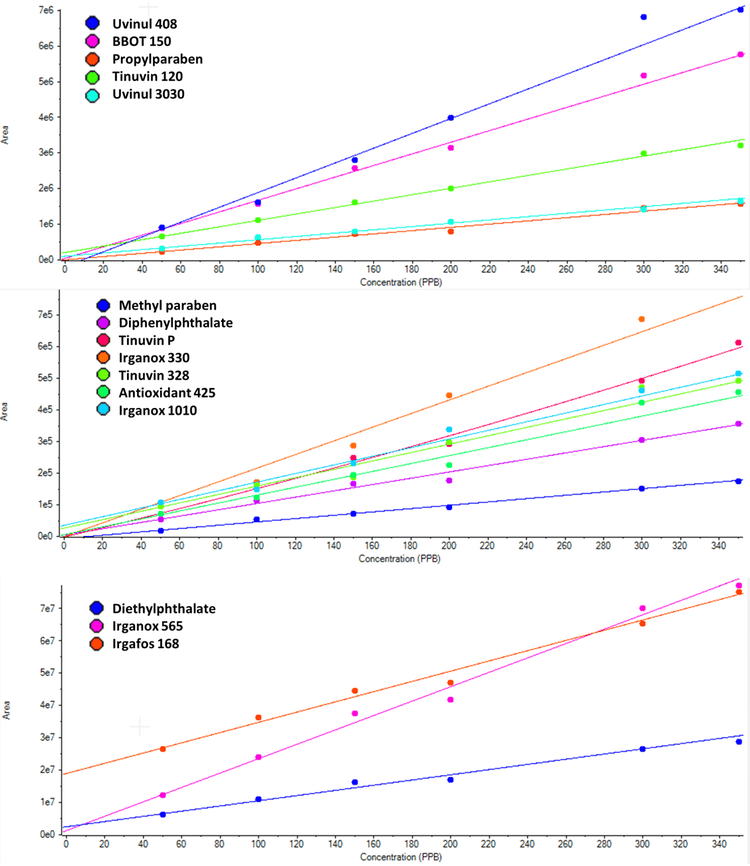
The limit of quantification is different for each analyte as expected as the MS response varies per analyte. The calibration range used here was from 50 to 400 ppb due to usual reporting range for E&L, however for some analytes the lower level of quantitation (LLOQ) might be lower. Figure 3 shows the XIC for each analyte at a concentration of 50 ppb. Figure 4 shows the calibration curves of all the analytes. The method is highly selective and sensitive using analyte specific MRM transitions for detection in matrix.
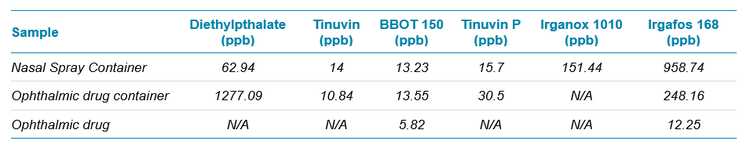
Next, the method was tested using a variety of drug products, nasal spray, ophthalmic drug container and drug product of the liquid formulation of the ophthalmic drug. The results are summarized in Table 1. Most of the E&L analytes were not within the detection range of the developed method. Only six E&L compounds (Diethylpthalate, Tinuvin 120, BBOT 150, Tinuvin P, Irganox 1010, Irgafos 168) were found in detectable range in these drug products. However, the detected amount was much less than analytical evaluation threshold specific for E&L testing.
Conclusions
The developed LCMS method for analysis of 15 E&L was successfully applied for analysis in pharmaceutical drug product and containers and closures. Further developments may include addition of more analytes to the method of analysis to result into broader area of application, like food, water, and environmental monitoring.
References
- USFDA, 21 CFR 211.94. cGMP for finished pharmaceuticals.
- Diane Paskiet, Dennis Jenke, Douglas Ball, et al. (2013) The Product Quality Research Institute (PQRI) Leachables and Extractables Working Group Initiatives for Parenteral and Ophthalmic Drug Product (PODP), PDA J Pharm Sci and Tech 67, 430-447.
- Dennis Jenke, Mitchell Poss, James Story, Alex Odufu, et al. (2014) Development and Validation of Chromatographic Methods for the Identification and Quantitation of Organic Compounds Leached from a Laminated Polyolefin Material, Journal of Chromatographic Science, 42, 388-395.
- Detailed Method information.
