Abstract
5-(4’-(azidomethyl)-[1,1’-biphenyl]-2yl)-1H-tetrazole (AZBT) is a known impurity found in sartan medications and there is some concern that AZBT could act as a mutagen. Here, an assay has been developed for the sensitive detection of the AZBT impurity in an irbesartan drug substance and a candesartan drug product using the QTRAP 4500 system. Excellent sensitivity was achieved with lower limits of quantification of 0.5 ng/mL which is well below the threshold of toxicological concern (TTC) for these drugs. With a total run time of 8 minutes, this robust method provides require sensitivity, linearity and recovery for assessing levels in APIs and drug products.

Introduction
In April 2021, AZBT, which is a known impurity in some sartan medications, tested positive on 2 independent Ames tests, suggesting that this compound might be a mutagenic impurity. Without further studies, it is paramount to monitor drug production and assess the levels of this compound in sartan medications to ensure that the threshold of toxicological concern (TTC) is not breached.1 TTC standards are outlined in the ICH M7 guidelines for the control of DNA-reactive (mutagenic) impurities in pharmaceuticals.2 Based on this regulation, long-term medications such as sartans, which are used to control high blood pressure, have a TTC of 1.5 µg per person per day.
Here, the levels of AZBT impurity were measured in 2 sartan medications. The drug substance, Irbesartan, was tested to determine impurities in an active pharmaceutical ingredient (API), whereas the drug product, Candesartan, was tested to determine impurities in a finished drug product.
According to the National Health Service, the largest dose for Irbesartan is 300 mg/day, whereas Candesartan has a maximum daily dose of 32 mg/day.3-4 Therefore, based on the 1.5 µg/day TTC for AZBT and the equation derived from Food and Drug Administration guidance,5 below, maximums of 5 µg/g and 47 µg/g AZBT are permitted in samples of Irbesartan and Candesartan, respectively.
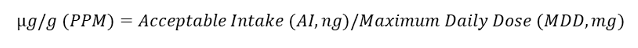
Key features of the QTRAP 4500 system for AZBT analysis
- Excellent sensitivity of the QTRAP 4500 system detected AZBT at an LLOQ value of 0.5 ng/mL in solution
- Quantification of AZBT was far below necessary limits outlined in the ICH M7 guidelines (1.5 µg/day)
- Reliable spike recovery was achieved for both the Irbesartan API and Candesartan tablet formulation, with recovery values between ± 15% of the expected amount
- Linearity was observed between 0.5 and 100 ng/mL, with an LLOD value of 0.1 ng/mL
- Increased specificity of analysis was achieved using 2 MRM transitions to provide ion ratio values.
Methods
Standard preparation: A stock solution of 100 µg/mL AZBT in methanol was purchased from Chiron. This solution was then diluted into 50:50, water/methanol to construct a calibration curve between 0.1 and 100 ng/mL.
Sample preparation: 10 mg of drug substance (Irbesartan API) or drug product (Candesartan tablet, crushed via mortar and pestle) was weighed into 5 mL Eppendorf tubes, to which 5 mL of 50:50 water/methanol was added via pipette. The resulting mixtures were vortexed for approximately 30 seconds to dissolve the Irbesartan or Candesartan, then were sonicated for 5 minutes. Finally, the solutions were centrifuged for 5 minutes at the highest centrifugation speed (10,416 rcf) and the supernatant was transferred to HPLC vials for analysis.
Spiked sample preparation: 10 mg of drug substance (Irbesartan API) or drug product (Candesartan tablet, crushed via mortar and pestle) was weighed into 5 mL Eppendorf tubes, to which 4.95 mL of 50:50 water/methanol was added via pipette. The Irbesartan mixture was spiked with 50 µL of a 1 µg/mL standard solution, whereas the Candesartan mixture was spiked with 50 µL of a 50 ng/mL standard solution. The resulting mixtures were vortexed for approximately 30 seconds to mix, then were sonicated for 5 minutes. Finally, the solutions were centrifuged for 5 minutes at the highest centrifugation speed (10,416 rcf) and the supernatant was transferred to HPLC vials for analysis. The final spiked samples contained either 10 ng/mL (5 µg/g) AZBT in Irbesartan or 0.5 ng/mL (0.25 µg/g) AZBT in Candesartan.
Chromatography: The ExionLC AD system was used with a Phenomenex Kinetex analytical column (XB-C18, 2.6 µm, 50 x 2.1 mm).
Mass Spectrometry: The QTRAP 4500 system, operating in positive ion mode and using electrospray ionization (ESI), was used for analysis. See Table 1 for MRM details.
Data processing: All data was processed using SCIEX OS software.
Results
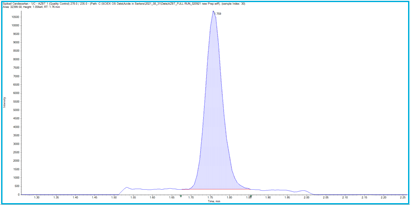
To assess this LC-MS method, several parameters were evaluated to determine its suitability for detecting and quantifying the AZBT impurity in both a sartan drug substance (Irbesartan API) and the final formulated tablet (Candesartan, Figure 1). The performance of the method was further assessed using an external standard calibration to evaluate the precision and accuracy of analysis when detecting AZBT externally versus when spiked into samples.
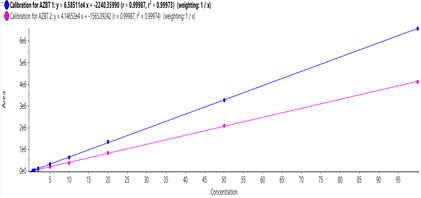
A calibration curve was constructed for the external standard, ranging in concentration from 0.1 to 100 ng/mL AZBT. The quantifier calibration curve was linear between 0.5 and 100 ng/mL, with an r value of 0.99987 (Figure 2). The calibration curve was used to determine an LLOD of 0.1 ng/mL and an LLOQ of 0.5 ng/mL for AZBT, as shown in Table 2. These values were reliable, based on the peak-to-peak signal-to-noise (S/N) ratio. The S/N ratios at the LLOD and LLOQ were 4.4 and 19.6, respectively. These ratios exceeded the minimum requirements of 3 for LLOD and 10 for LLOQ.
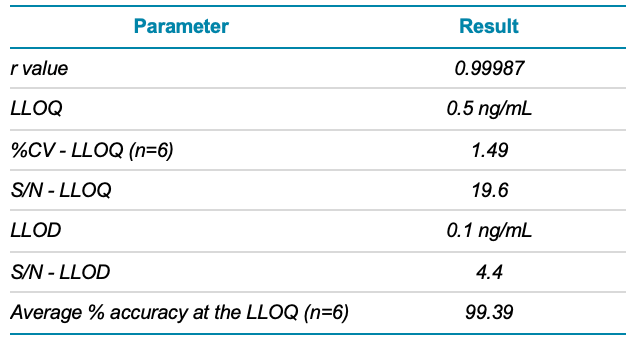
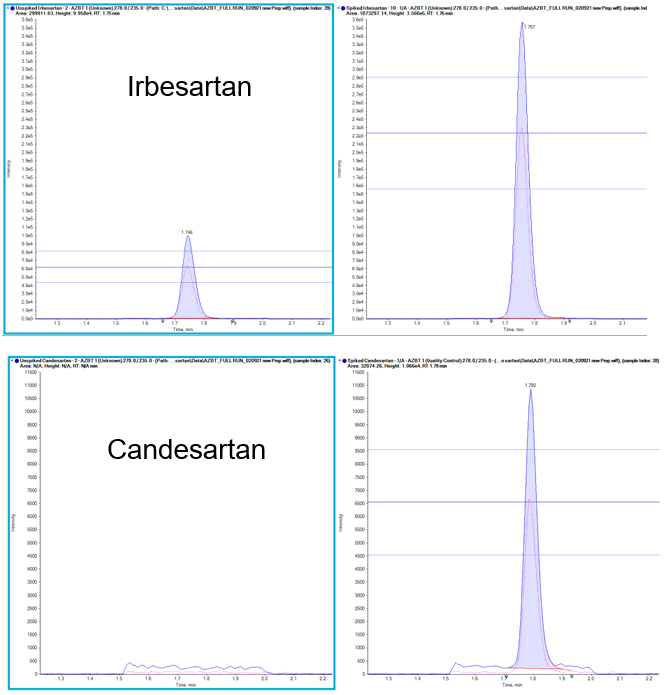
Precision was assessed at the LLOQ by performing 6 injections and calculating the %CV of the LLOQ concentration. As shown in Table 2, for this application, the %CV at the LLOQ was 1.49, indicating that the measurements were highly precise. Moreover, an average accuracy of 99.39% was calculated for the 6 replicate injections analyzed, over a range of 95.60 to 105.98%. Both precision and accuracy values calculated were within acceptable criteria, as a %CV <10 and accuracy within ± 30% of the expected value are considered typical.
When analyzing complex mixtures, such as a final tablet formulation, it is necessary to maximize the specificity of the LC-MS/MS analysis. To do so, 2 MRM transitions were used with the parameters outlined in Table 3. The ion ratios for the AZBT-spiked samples are similar to the ratios observed in the standard solution, indicating that there is minimal interference in the MRM signals (see Figure 3 which highlights the ion ratio lines used).
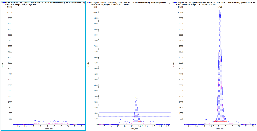
Figure 4 shows the overlaid extracted ion chromatograms (XICs) of the quantifier and qualifier ion with added ion ratio lines to indicate the set tolerance (± 20 %). This analysis is easily performed in the Analytics module of the SCIEX OS software, which flags samples if the ion ratio tolerances are exceeded. This function indicates potential interference and signals a need for data review
Both sample types (Irbesartan API and Candesartan tablet) were initially spiked at the LLOQ of the method (0.5 ng/mL or 0.25 µg/g). Under these conditions, a large amount of AZBT was detected in the un-spiked Irbesartan API sample (see Figure 4), indicating that a larger AZBT spike was required. Therefore, a 10 ng/mL (5 µg/g) spike concentration was used to ensure that the spike recovery of the method could be accurately assessed.
To evaluate the spike recovery of the method, the Irbesartan and Candesartan samples were each prepared 4 times (2 un-spiked and 2 spiked). Each un-spiked sample was injected in duplicate, and the spike preparations were injected in triplicate to determine precision. Table 4 provides an overview of the amount of AZBT detected, %CV values and spike recovery values determined for each sample type. Parameters of <10% for precision (%CV) and accuracies between ± 30% of the expected value were achieved. This is shown in Figure 4, which compares an example of an un-spiked and spiked sample for both Irbesartan and Candesartan.
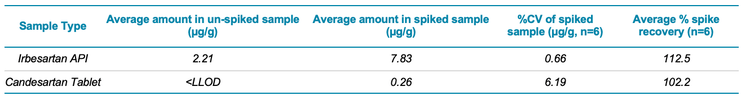
Conclusions
- A method suitable for the analysis of AZBT in both an Irbesartan API and Candesartan tablet was developed
- An LLOD value of 0.1 ng/mL and an LLOQ value of 0.5 ng/mL highlight the excellent sensitivity provided by the QTRAP 4500 system
- A linearity spanning from 0.5 to 100 ng/mL with an r value >0.99 demonstrates accurate quantification across this range
- Spike recovery was reliable for both Irbesartan and Candesartan samples, with accuracy values between ±15% of the expected amount
- The use of ion ratio values increased specificity and ensured accurate quantification in matrix
- Custom calculations created in the SCIEX OS software supported all data processing on a single platform.
References
- CMDh/430/2021 – request for investigation – April 2021
- ICH guideline M7(R1) on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk - February 2018
- NHS – Medicines A to Z – Candesartan
- NHS – Medicines A to Z – Irbesartan
- Control of Nitrosamine Impurities in Human Drugs – FDA - February 2021
