Abstract
Here, a LC-MS method for the analysis of eight nitrosamine compounds is described, with a lower limit of quantitation (LLOQ) of 0.05 µg/g in a drug product which corresponds to significantly less than the TTC for most pharmaceutical products.
Introduction
Genotoxic impurities (GTI’s) are intermediate, reactive products or degradants formed during drug synthesis, formulation or storage. These impurities can damage human genetic material at very low levels, leading to DNA mutations which can contribute to tumorigenesis and carcinogenicity. Because of these compounds’ potencies, they pose a threat to the safety of medication. To prevent large-scale recall incidents, such as those involving nelfinavir mesylate (Viracept) in 2007, or valsartan (Diovan) in 2018, regulatory agencies including the US FDA and the European Medicines Agency (EMA), have issued guidelines on the allowable limits of genotoxic impurities in pharmaceutical products to ensure their safety. These guidelines state exposure to GTI’s must be below the threshold of toxicological concern (TTC) of 1.5 mg per day.
Nitrosamines are some of the most potent and well-studied chemical carcinogens. They are commonly found in grains, cured meats, beer, tobacco and drinking water as well as being intermediates in organic synthesis. Due to their potent genotoxicity, it is important to accurately quantitate this group of compounds in pharmaceutical ingredients and products during drug development. However, it can be challenging to develop a robust, sensitive analytical method for them due to their low molecular weight and high hydrophilicity.
In this publication, a method for the analysis of eight nitrosamine compounds is described, with a lower limit of quantitation (LLOQ) of 0.05 mg/g in a drug product which corresponds to significantly less than the TTC for most pharmaceutical products.
Key features of the SCIEX Triple Quad 4500 system with ExionLC system
- The SCIEX Triple Quad 4500 system is industry proven to be a sensitive and robust sensitivity for quantitaiton
- The ExionLC system pairs seamlessly with SCIEX mass spectrometers providing a complete, workhorse LC-MS/MS solution
Methods
Sample preparation: Samples were prepared by dissolving the final drug product, in this case, the contents of an 80 mg capsule, by emptying the contents and adding 40 ml of 1:1 methanol:Water, bringing the concentration to 2 mg/ml. The mixture was vortex mixed for 1 minute followed by sonication in a bath sonicator for 20 minutes. The solution was allowed to settle for 1 hour at room temp and the supernatant was transferred and centrifuged for 5 minutes at 14k RPM. Aliquots of the solution were transferred to HPLC vials for analysis by LC-MS/MS. Standards are prepared by diluting mixtures of the eight compound’s working standards into the extraction solvent to final concentrations of 0.1, 0.2, 1.0, 2.0, 5.0, 10 and 20 ng/ml of each analyte prior to mixture and transfer. The 0.1 ng/mL in the extract corresponds to 0.05 mg/g in the tablet.
Analytical conditions: A SCIEX ExionLC system and SCIEX Triple Quad 4500 system were used for sample analysis, with a Phenomenex Kinetex F5, 2.5mm, 50x2.1 mm HPLC column for separation.
- Mobile phase A: Water with 0.1% formic acid by volume
- Mobile phase B: Methanol
- Total flow rate: 0.5 ml/min
- Column temp: 40oC
- LC Gradient is specific in Table 2.
The mass spectrometer was operated in positive MRM mode using APCI ionization.
- Nebulizer current: 3mA
- Curtain Gas: 30 psi
- GS1: 35 psi
- CAD: 8
- Temp: 350oC
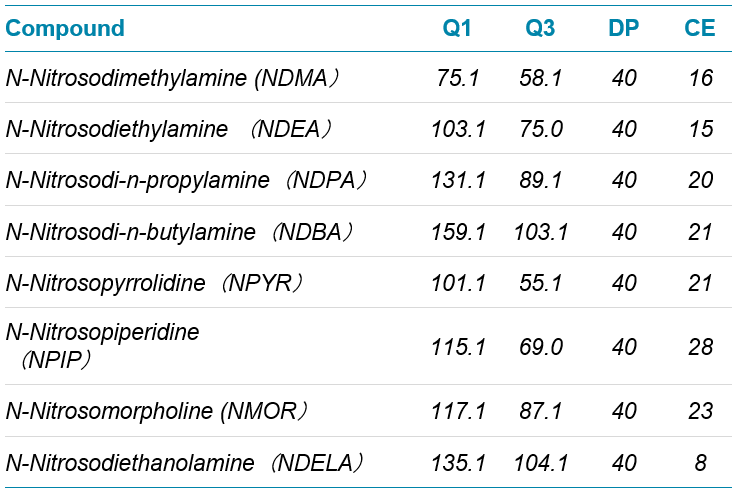
- MRM transitions are specified in Table 1.
Results
Properties of the analysis were evaluated to ensure its suitability for adoption for quantitation.
Specificity
A valsartan standard was dissolved to 2 mg/mL and spiked with working solutions of the eight nitrosamines at 0.5ng/mL, 5.0ng/mL and 15 ng/mL to make solutions equivalent to 0.25, 2.5 and 7.5 mg/g API samples. An additional sample was prepared by dissolving a Sultan (diphenhydramine) capsule to a 2.0 mg/mL concentration. The samples were then prepared as described and analyzed by LC-MS/MS. Responses for all nitrosamine spiked samples were linear, with no interferences in the blank. The sultan sample showed response for n-nitrosodimethylamine that quantitated to 0.2 ng/mL (shown in Figure 1 below), the equivalent of 0.1 mg/g in the drug product – which is below the threshold of toxicological concern.
None of the other compounds were observed in the sample, or the blank.
Linear dynamic range
Working standard solutions were prepared by serial dilution of the primary stock solution in the sample dilution buffer to 0.1, 0.2, 1.0, 5.0, 10 and 20 ng/mL.
As seen in Figure 2 below, the response for all eight compounds is linear over the calibration range.
Recovery and reproducibility
Recovery and reproducibility were evaluated for the QC samples described in the Specificity section. The recovery of all eight compounds in the three QC levels fell between 89.5% and 112.0%, with %RSD (n=6 at each level) between 0.61% and 4.42%. Data is summarized in Table 3. The %RSD (n=6) was evaluated at the LLOQ (0.1 ng/mL) and for the eight compounds which fell between 1.53% and 2.48%, is summarized in Table 4.
Summary
In this publication, a 6.5-minute LC-MS/MS method has been described for the quantitation of eight nitrosamine compounds in final drug product using the SCIEX Triple Quad 4500 System coupled with an ExionLC system. The results demonstrate good specificity, with a linear dynamic range from 0.1 to 20 ng/mL (correlation coefficient >0.998). The LLOQ of 0.1 ng/mL is equivalent to 0.05 ug/g of impurity in the drug product, which is lower the threshold of toxicological concern defined by the EMEA and USFDA.
Related content
- Analysis of Genotoxic Nitrosamines in Losartan and Ranitidine Active Pharmaceutical Ingredients. SCIEX technical note, RUO-MKT-02-11183-A.
- Highly Selective and Sensitive Method for Quantitation of Nitrosamines in Valsartan Drug Substance. SCIEX technical note, RUO-MKT-02-11212-A.
- Analysis of genotoxic nitrosamines in losartan using the SCIEX Triple Quad 7500 system. SCIEX technical note, RUO-MKT-02-13005-A.






