Abstract
Recently, certain Valsartan drugs have been recalled due to contamination with N-nitrosodimethylamine (NDMA), which is a potential human carcinogen. Here, an LC-MSMS method was developed and partially validated for the quantitative analysis of six nitrosamines. Good linearity, sensitivity and reproducibility was achieved over a broad concentration range, meeting regulatory requirements.

Introduction
Valsartan and other related “sartan” drugs are used to treat patients with hypertension (high blood pressure), and those with heart failure or who have had a heart attack (Figure 1). The mechanism of action of “sartans” is to block the action of angiotensin-II, a hormone that constricts blood vessels and causes blood pressure to rise.
In July of 2018, the US FDA issued a recall notice for a lot of the generic angiotensin II receptor blocking drug, valsartan, that was manufactured at a facility in China, because of contamination with unacceptable levels of the genotoxic compound n-nitrosodimethylamine (NDMA). In the years since, there have been hundreds of recalls issued for various pharmaceuticals, including many lots of valsartan, because of nitrosamine contamination. In addition to the unknown effects of patient exposure to these impurities, it’s not possible to accurately calculate a financial cost of these recalls, which could be well into the millions of US dollars. In this publication a method for the detection and quantification of six common nitrosamine compounds is described that is capable of robustly and accurately monitoring these important contaminants at levels below regulatory action limits.
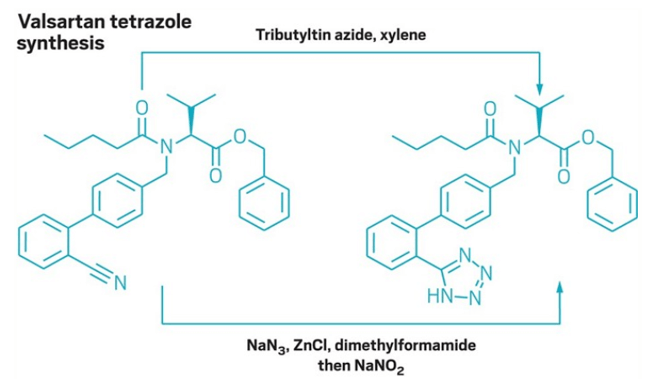
Nitrosamine contamination can have multiple sources. It has been shown to arise from the unintentional use of contaminated ingredients or equipment, and nitrosamines can also be formed as side products of certain synthetic chemical reactions. In at least one case, NDMA was thought to be introduced when changing the synthetic pathway of valsartan in order to improve production efficiency, see Figure 1.1
The method described here is specific for six nitrosamines, see Table 1, and it is able to accurately detect and quantify them all at levels well below current FDA action limits in valsartan active pharmaceutical ingredient (API). This should allow for the method to be applicable in the future when action levels are lowered, as has been discussed by regulatory agencies.2
Key features of targeted quantitative nitrosamine LC-MS/MS method
- Simple sample preparation used only dissolution in methanol
- Optimized chromatography separates the six nitrosamine impurities away from the valsartan, ensuring good sensitivity of impurity detection
- SCIEX Triple Quad 5500+ System provides the sensitivity required for detection of the six nitrosamine impurities
- Extended linear dynamic range of the system ensures detection of low level impurities in the presence of high API concentrations
Methods
Sample preparation:API was weighed and completely dissolved by vortexing in methanol to a concentration of 100 mg/mL.
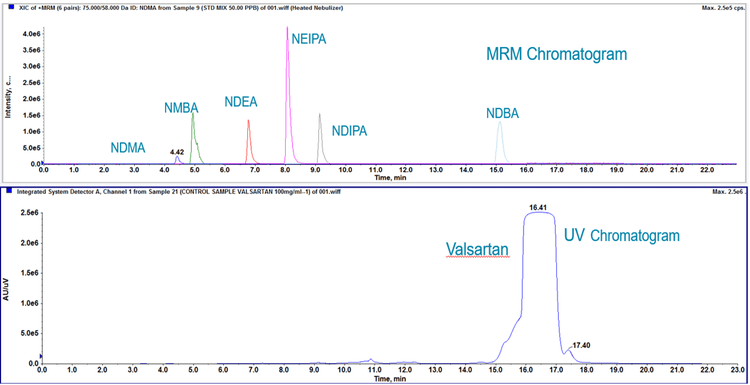
Chromatography:The gradient was developed to separate all of the analytes in the panel from the valsartan API to prevent any ionization suppression from occurring, and includes a divert step to prevent large amounts of the API from contaminating the mass spectrometer source. An example chromatogram is shown in Figure 2. Please see the Supplementary Information for details of the chromatography, including gradient profile, column and mobile phases.3
Mass spectrometry:The SCIEX Triple Quad™ 5500+ System - QTRAP® Ready was operated in positive atmospheric pressure chemical ionization (APCI) mode using Analyst® Software 1.7.1. The MRM compound parameters (DP, EP, CE, CXP) were optimized for nitrosamines, the source parameters were also optimized. Details can be found in the Supplementary Information.3
Standard preparation:1.0 mg/mL stock solutions in MeOH were stored at 20 ºC until use. Working standards were prepared by serial dilution from stock in 10% MeOH in water to concentrations of 500 to 0.5 ng/mL. The lowest calibration point corresponds to 0.005 ppm (5ng/g) with regard to the test article.
Data processing:MultiQuant™ Software 3.0.3 was used for data analysis. A 1/x2 weighted linear regression was used to calculate the concentrations.
Results
Time was spent on optimizing the chromatography such that the valsartan API is separated from all 6 nitrosamines. Using the established gradient, good separation is obtained (Figure 2). Because of the high level of API, it is critical to achieve separation from the impurities to reduce risk of ion suppression.
Linearity, precision and accuracy were all evaluated as part of the method verification. Recovery in matrix was evaluated at the limit of detection, limit of quantification and at the FDA specified limit of daily exposure (0.03 ppm in API).
Linearity for all six compounds was good over the nine point range of 0.5 to 500 ng/mL, with all compounds showing >0.99 r2 correlation values.
Recovery and reproducibility data for the LOD, LLOQ and specification level spikes in the valsartan matrix are shown in Table 2. It’s worth noting that even though reagent grade API was used for this analysis, five of the six nitrosamine compounds in this panel were found in the API. Because of this, standard addition quantification was used to calculate the recovery.
Conclusions
The method described here is capable of accurately detecting and quantifying the six nitrosamines in valsartan API at levels well below the current FDA specifications. Implementation of this method on the SCIEX Triple Quad 5500+ System - QTRAP Ready will enable the monitoring of the six listed nitrosamine compounds to help ensure fewer costly product recalls, and unnecessary patient exposure to these genotoxic compounds.
References
- American Chemical Society, C&EN news, “A side reaction may have led to impurities found in valsartan heart drugs“, Published online, Feb 19, 2019.
- US Food and Drug Administration website.
- Download supplementary method information
Related content
- Analysis of Genotoxic Nitrosamines in Losartan and Ranitidine Active Pharmaceutical Ingredients. SCIEX technical note RUO-MKT-02-11183-A.
- Rapid Analysis of Genotoxic Nitrosamines by HPLC-MS-MS. SCIEX technical note RUO-MKT-02-9127-A.

