Abstract
Detection of genotoxic nitrosamines in various drug products over the last few years has driven the need for analytical techniques that can quantify these compounds at very low levels to maintain the safety of pharmaceutical products. Here, an assay was developed on the QTRAP 6500+ System for the quantitation of 8 nitrosamines. In addition, MRM triggered MS/MS was performed during the assay to allow for confident confirmation of each of the nitrosamines.
Introduction
In 2018, it was found that a number of Angiotensin II receptor blockers (ARBs) which includes various ‘sartan’ products were possibly contaminated with nitrosamine impurities. In addition, nitrosamines were found in other products such as ranitidine (a treatment for heart burn and stomach ulcers) seemingly suggesting the problem was larger than first envisioned. As a result of this, numerous drug products were recalled.
Due to the genotoxic nature of nitrosamines it was paramount that an analytical method be created that was both highly sensitive and qualitatively robust to ensure correct identification and quantification of the impurities. This analytical testing is now performed before medications are released so that human contact with these compounds is limited.
Here, a quantitative method for nitrosamines has been developed using the SCIEX QTRAP 6500+ LC-MS/MS System.
This system was chosen because it is highly sensitive and allows for ultra-low-level trace analysis. Unique to this assay is the use of the linear ion trap functionality in the QTRAP System, which can provide full scan MS/MS data within the same injection as a traditional MRM analysis. Combined with library searching using a curated nitrosamine library and SCIEX OS Software, a highly quantitative assay with confident compound confirmation has been demonstrated (Figure 1).
Key features of SCIEX QTRAP 6500+ LC-MS/MS System for trace detection
- Delivers ultra-low-level MRM detection allowing for LLOQ values which are far improved on current regulations
- Unique triple quadrupole linear ion trap configuration enables qualitative full scan MS/MS to be obtained for each detected MRM signal for compound confirmation with high confidence
- Efficient and accessible data processing using SCIEX OS Software including library search technology for compound confirmation
Methods
Standard Preparation: The nitrosamine standard mixture was obtained from LGC. The standard was dissolved and diluted in water to obtain standards covering a range of 2.5 – 5000 pg/mL.
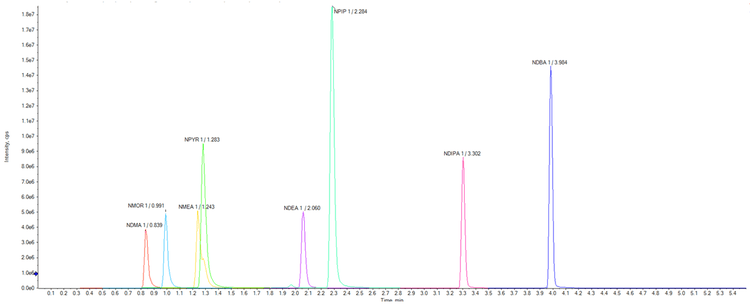
Chromatography:Chromatographic separation was performed using the ExionLC™ AD System which provides very low carryover and full UHPLC capabilities. The column used was a Phenomenex Luna Omega C18 1.6µm, 100 x 2.1mm which provided very good separation of the eight compounds (Figure 2). Details of the chromatography are outlined in the Supplementary Information.5
Mass Spectrometry: These experiments were performed using the SCIEX QTRAP® 6500+ LC-MS/MS System. The system was operated in positive ionization mode using the atmospheric pressure chemical ionization (APCI) probe on the IonDrive™ Turbo V Ion Source. Data was acquired using Analyst® Software. Details for MS conditions are outlined in the Supplementary Information.5
Data Processing: Data was processed using SCIEX OS Software and LibraryView™ Software.
Quantitative analysis
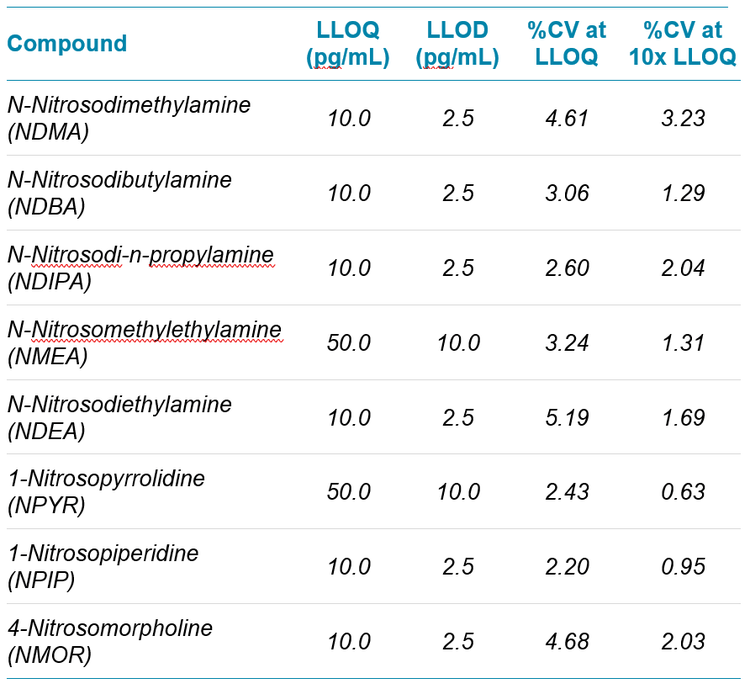
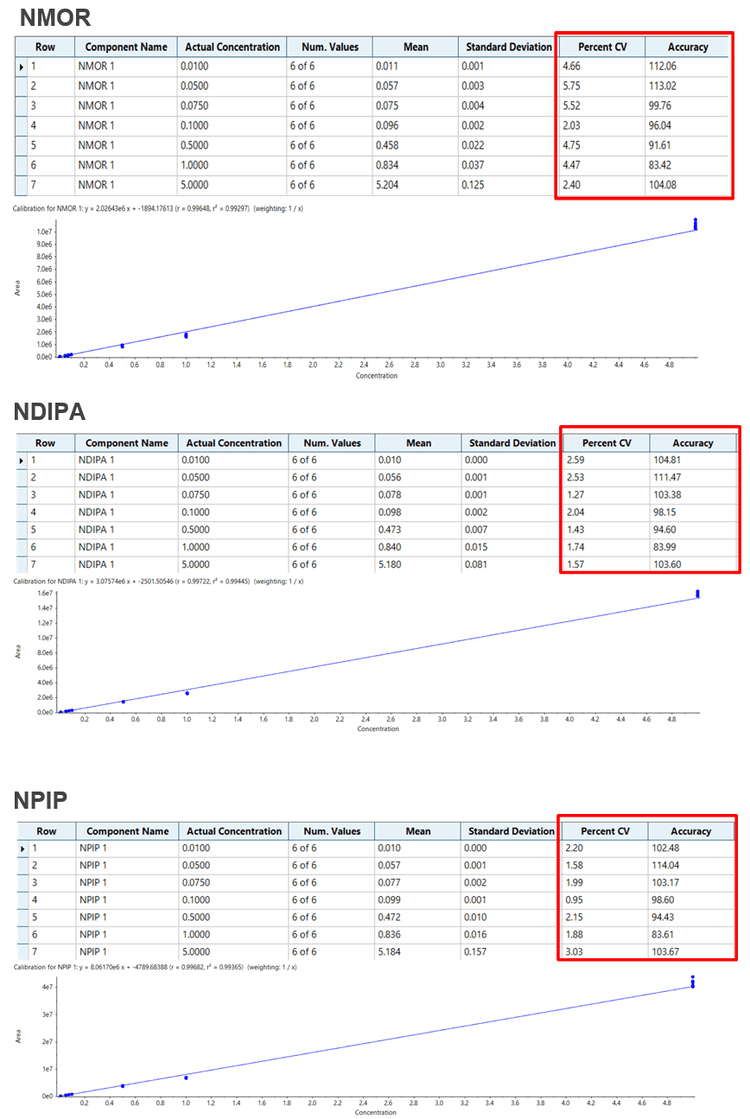
Impressive sensitivity was obtained using the optimized assay on the QTRAP 6500+ LC-MS/MS System. Table 1 highlights the LLOQ values obtained for all eight nitrosamines tested. The %CV was also calculated which illustrates that the instrument is not just sensitive but provides a high level of precision as well.
Compound confirmation with QTRAP System technology
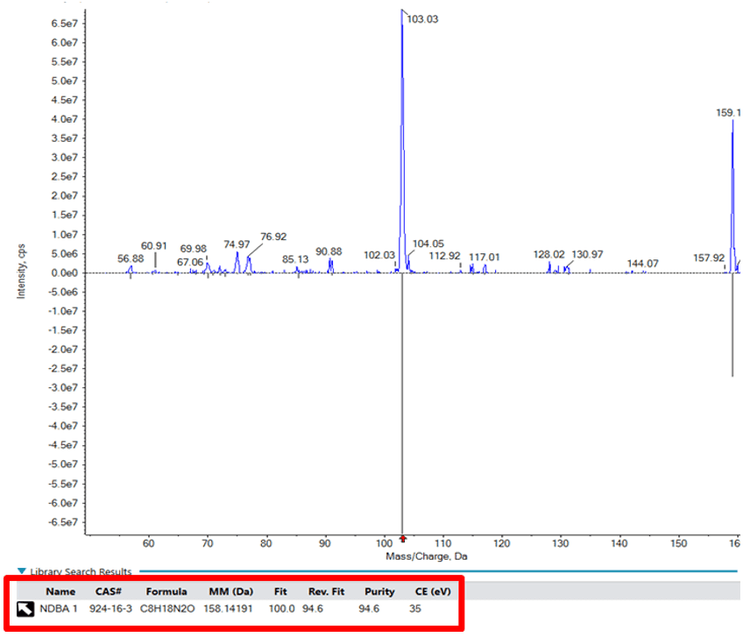
QTRAP System technology allows the acquisition of full scan MS/MS simultaneously with an MRM experiment mode. This MS/MS can be searched against a compound library for an orthogonal confirmation of identity beyond the detection of an MRM signal.
For this assay, a library of relevant nitrosamine impurities was created using SCIEX OS Software and LibraryView Software based on data from nitrosamine standards. This library was then used in subsequent assays to confirm the identity of the nitrosamines detected by the MRM quantitative assay. This ensures that the correct analyte is being measured and helps rule out any artefact peaks that may occur.
Conclusions
Here a highly sensitivity assay for the detection of nitrosamines has been demonstrated using the QTRAP 6500+ LC-MS/MS System. The lower limits of quantification were 10 pg/mL for most compounds, with excellent reproducibility and accuracy. The calibration curves were evaluated across a range of 10 to 5000 pg/mL and demonstrated very good linearity. Note the assay has been tested here in buffer, next steps are to obtain active pharmaceutical ingredients (APIs) and test detection limits in matrix.
MRM signal is often sufficient for detection of compounds but higher confidence that the correct compound has been detected can be achieved through the acquisition of full scan MS/MS on the compound. This is easily done in a single injection using the QTRAP System’s unique configuration. MRM triggered MS/MS acquires high quality MS/MS that can be used post-acquisition for compound confirmation. This simultaneous quantitative and qualitative analysis was demonstrated and has been shown to be highly successful.
Using the integrated data processing power of SCIEX OS Software, both the quantitative MRM data and the qualitative MS/MS data can be processed within a single software. The AutoPeak integration algorithm was used for automatic MRM integration with minimal need for re-integration before statistical analysis was then performed on the results. Library searching against a curated nitrosamine library confirmed the identity of all quantified nitrosamines.
References
- Food and Drug Administration, FDA announces voluntary recall of several medicines containing Valsartan following detection of an impurity, 13th July 2018.
- European Medicines Agency, EMA advises companies on steps to take to avoid nitrosamines in human medicines, 26th September 2019.
- Next Generation Quality Control in Pharma Applications. SCIEX Technical note RUO-MKT-02-10464-A.
- Highly selective and sensitive method for quantitation of Nitrosamines in Valsartan Drug substance. SCIEX Technical note RUO-MKT-02-11212-A.