Quantifying fluticasone propionate and salmeterol xinafoate with high sensitivity in human plasma
Analysis of highly potent inhaled compounds using the SCIEX Triple Quad 7500 system
Rahul Baghla, Santosh Kumar Kapil, Rolf Kern
SCIEX, USA
Abstract
Drugs with high potency are dosed at lower levels and hence have lower circulating concentrations in plasma. This drives the requirement for increasingly sensitive assays to accurately and robustly quantify these drugs to understand pharmacokinetics. Here, an LC-MS/MS method has been developed two quantify two inhalants, fluticasone propionate and salmeterol xinafoate, in a single 8 minute method in human plasma. Leveraging the sensitivity of the SCIEX Triple Quad 7500 System, these drugs were quantified with LLOQs in the 0.05 pg/mL range using 400 uL of plasma.

Introduction
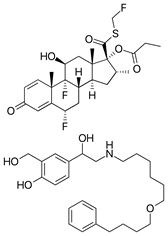
Fluticasone propionate is a synthetic trifluorinated glucocorticoid with potent and generalized anti-inflammatory activity. It is currently one of the most effective drugs used in the treatment of asthma. Salmeterol xinafoate is a long-acting β-adrenergic receptor antagonist with very high selectivity for the β2-receptor subtype, which produces airway smooth muscle relaxation and consequently bronchodilation.1 For patients with persistent asthma, inhaled corticosteroids (ICS) have been the first-line treatment regardless of disease severity. Considering the guidelines, asthma sufferers whose condition is not sufficiently controlled with ICS alone are recommended to include a long-acting β2-agonist (LABA) in their treatment. Salmeterol is a selective LABA, which causes bronchodilation and inhibition of the release of hypersensitivity mediators from mast cells. The corticosteroid fluticasone propionate inhibits eosinophil activation and the subsequent release of inflammatory mediators.2
The lowest daily pediatric dose of fluticasone propionate and salmeterol xinafoate for children 12 years and older is 90 micrograms and 42 micrograms, respectively, for the aerosol and 100 micrograms and 50 micrograms, respectively, as an inhalation powder.5 This leads to very low circulating levels of plasma concentrations of these drugs in human blood. Fluticasone propionate concentrations in plasma following administration of fluticasone propionate in the form of an aqueous nasal spray are 10.8 pg/mL to 14.1 pg/mL.5,7
Therefore, it is very important to have an analytical method with ultra-low detection limits for both analytes in human plasma to understand pharmacokinetics. Here, using a smaller sample volume6 than was previous possible, an LC-MS/MS method has been developed for the ultra-low level quantification of inhalants in human plasma using the SCIEX Triple Quad 7500 system with fluticasone propionate and salmeterol xinafoate as model compounds.
Figure 1. Fluticasone propionate and salmeterol xinafoate structures.
Key features of the SCIEX 7500 system for quantification of low-level inhalants
- The SCIEX 7500 system provides high sensitivity for bioanalytical quantification with improvements in ion generation due to the OptiFlow Pro ion source with E Lens probe, and improvements in ion sampling, due to D Jet ion guide8
- SCIEX OS software provides an easy-to-learn, easy-to-use, all-in-one platform for acquisition
- High sensitivity of the SCIEX 7500 system enables detection at ultra-low levels of fluticasone propionate and salmeterol xinafoate in low volume of human plasma
- Loading lower on the column matrix enables opportunities to reduce run time for high-throughput sample analysis
Methods
Sample preparation: Fluticasone propionate and salmeterol xinafoate were spiked in 400 µL of human plasma aliquots in the range of 0.050 to 50.000 pg/mL, with 5 pg of internal standard. Samples were extracted using protein precipitation with 500 µL of 0.2 M zinc sulfate, samples were vortexed and centrifuged at 6000 rpm for 5 minutes followed by SPE using supernatant liquid with phenomenex C18-SPE cartridges. Cartridges were conditioned using 1 mL of methanol followed by 1 mL of water. The sample was loaded onto the cartridge and followed by a series of washes: water, then 25% methanol in water. Analytes were eluted using acetonitrile and dried under a nitrogen stream at 40°C. Samples were reconstituted with 100µL of 60% mobile phase A and 40% mobile phase B, then analyzed using LC-MS/MS.3
Chromatography: Samples were analyzed using the ExionLC AD system at a flow rate of 0.3 mL/min using Phenomenex Kinetex 1.7µm EVO C18 100 Å, LC column 100 x 2.1 mm with a 8-minute gradient (Table 1). 10 µL injection volume was used for analysis.
Table 1. Chromatographic gradient.
Mass spectrometry: Samples were analyzed using the SCIEX 7500 system equipped with the OptiFlow Pro ion source, and the system was controlled by SCIEX OS software. The optimized MS parameters are listed in Table 2.
Data processing: Data processing was done using SCIEX OS software using the Analytics module.
Table 2. Optimized MS parameters.
Quantification results
Calibration curves were acquired across the concentration range of 0.050 to 50.00 pg/mL. Each concentration was analyzed in triplicate in order to assess method reproducibility. The resulting curves are shown in Figure 2 and Figure 3. Very good linearity was observed across the concentration ranges analyzed. The quantification results are summarized in Table 3. Samples analyzed in triplicate showed good reproducibility. Excellent %CV were achieved across all concentration levels with no interference in blank human plasma samples for fluticasone propionate and salmeterol xinafoate. The method provided a lower limit of quantification (LLOQ) of 0.050 pg/mL for fluticasone propionate and salmeterol xinafoate, with 0.4 mL of human plasma and a total run time of 8 minutes.
Figure 2. Calibration curve for quantification of fluticasone propionate in human plasma. Good linearity was observed for the sample concentrations from 0.050 to 50 pg/mL, with a regression coefficient (r) of 0.9973 using weighting of 1/x2. |
Figure 3. Calibration curve for quantification of salmeterol xinafoate in human plasma. Good linearity was observed for the sample concentrations from 0.050 to 50 pg/mL, with a regression coefficient (r) of 0.9971 using weighting of 1/x2.
As summarized in Table 3, the assay accuracy for fluticasone propionate was between 93.16% and 106.87%, and all %CV were below 10%. As shown in Table 4 for salmeterol xinafoate, the assay accuracy was between 94.91 and 102.75% and all %CV were below 13%. Both assays provided accuracy and reproducibility well within acceptance criteria for all tested samples.
The extracted ion chromatograms (XICs) for the observed LLOQs for each compound are shown in Figure 4 and 5. The double blank and blank injections are also provided for comparison. In both cases the blank is clean and there is a very distinct peak at the LLOQ.
Current results were achieved using plasma sample volumes of 400 µL and results demonstrate that very good LLOQs and reproducibility were achieved.
Table 3. Quantification summary for fluticasone propionate.
Table 4. Quantification summary for salmeterol xinafoate.
Figure 4. XIC of MRM transition for fluticasone propionate. Double blank and blank from extracted plasma (A and B) and LLOQ at 0.050 pg/mL (C) and 0.100 pg/mL (D).
Figure 5. XIC of MRM transition for salmeterol xinafoate. Double blank and blank from extracted plasma (A and B) and LLOQ at 0.050 pg/mL (C) and 0.100 pg/mL (D).
Conclusions
Here, a highly sensitive and reproducible assay for the quantification of both fluticasone propionate and salmeterol xinafoate in human plasma has been developed on the SCIEX 7500 system.
- Lower limits of quantification of 0.050 pg/mL for fluticasone propionate and salmeterol xinafoate were achieved. This was done using smaller sample volumes than previously described.7
- The method showed very good reproducibility and %CV for triplicate injections at all concentration levels
- The method demonstrated the ability to routinely detect ultra-low levels of both analytes in a 8-minute run time, which will allow bioanalytical labs to deliver high-quality data with good throughput
References
- Silvestro L, Savu SR, Savu SN, Tudoroniu A, Tarcomnicu I. (2012) Development of a sensitive method for simultaneous determination of fluticasone propionate and salmeterol in plasma samples by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. May;26(5): 627-35.
- Garg M, Naidu R, Birhade A, Iyer K, Jadhav R, et al. (2017) Bioequivalence of Two Formulations of Salmeterol Xinafoate/Fluticasone Propionate HFA pMDI in Healthy Volunteers. J Bioequiv Availab 9: 536-546. doi: 10.4172/jbb.1000359
- Ji, Allena & Zhou, Dawei & Zhang, Shu & Cawley, Monica & Fang, Xinping & Wu, Jinn. (2013). Ultrasensitive and automated I pg/ml fluticasone propionate assay in human plasma using LC-MS/MS. Bioanalysis. 5. 423-35. 10.4155/bio.12.338.
- Clinical Pharmacology review – Fluticasone Furoate, NDA 205625.
- Fluticasone dosage, Sep 23, 2020
- Harrison TW, Tattersfield AE. (2003) Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003 Mar;58(3): 258-60.
- Highly sensitive LC-MS/MS method for the quantification of fluticasone propionate in human plasma. SCIEX technical note, RUO-MKT-02-6510-B.
- Enabling new levels of quantification. SCIEX technical note, RUO-MKT-02-11886-A.
 Click to enlarge
Click to enlarge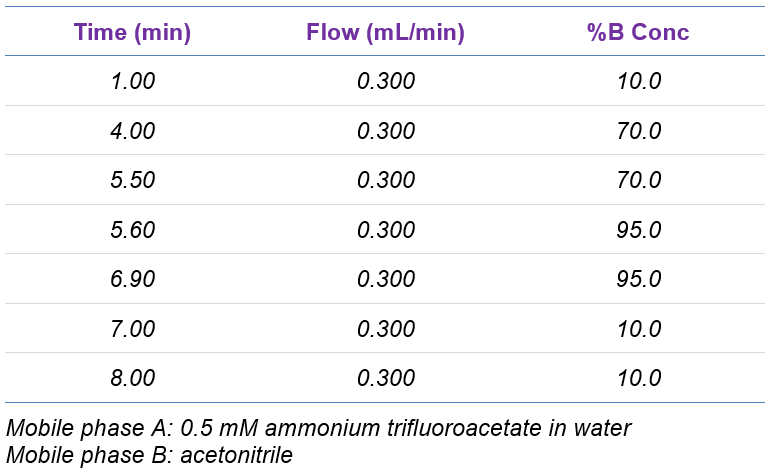 Click to enlarge
Click to enlarge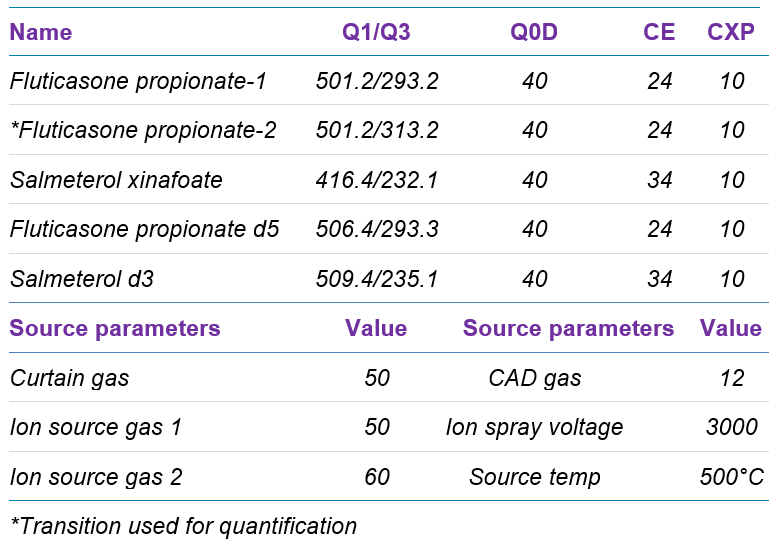 Click to enlarge
Click to enlarge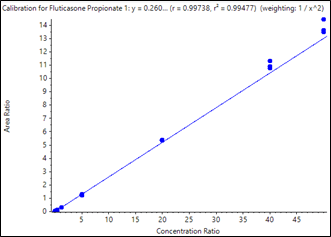 Click to enlarge
Click to enlarge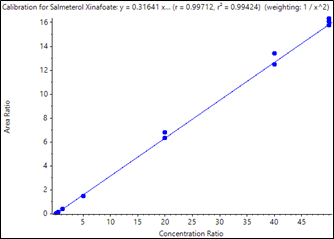 Click to enlarge
Click to enlarge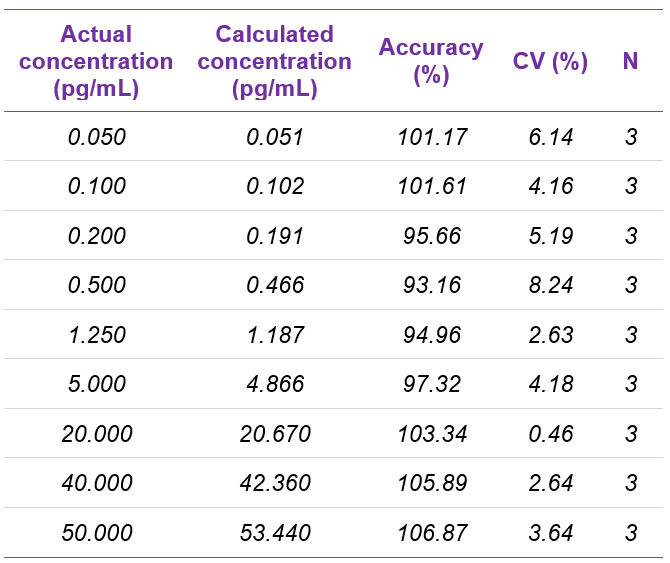 Click to enlarge
Click to enlarge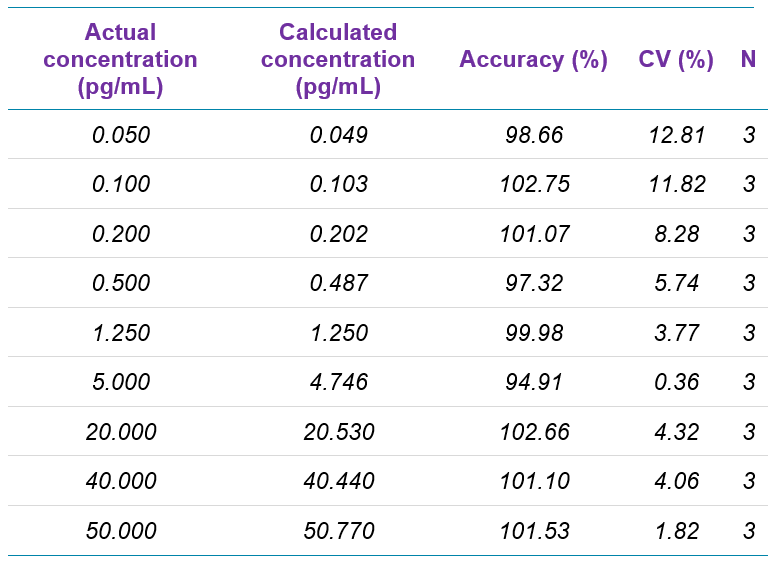 Click to enlarge
Click to enlarge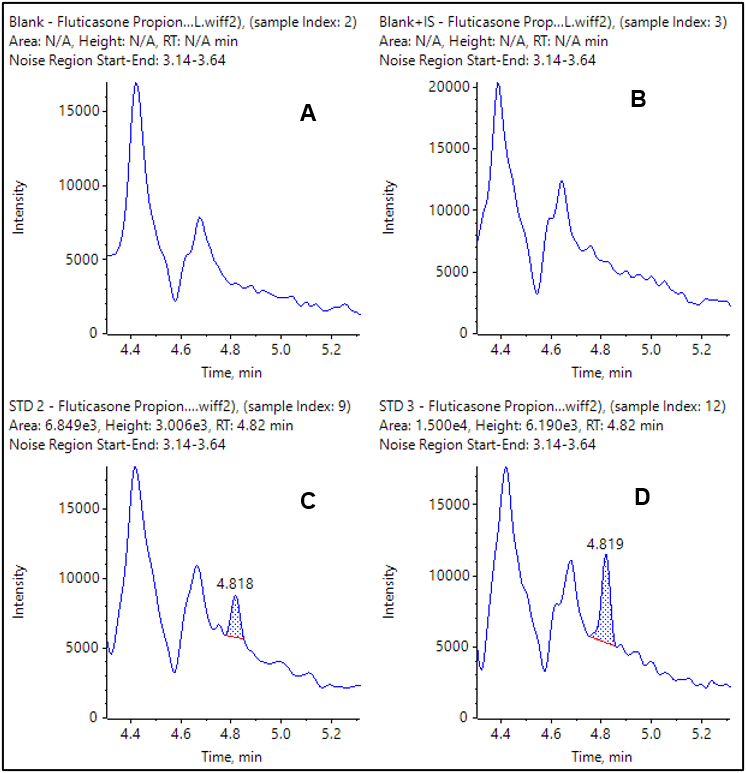 Click to enlarge
Click to enlarge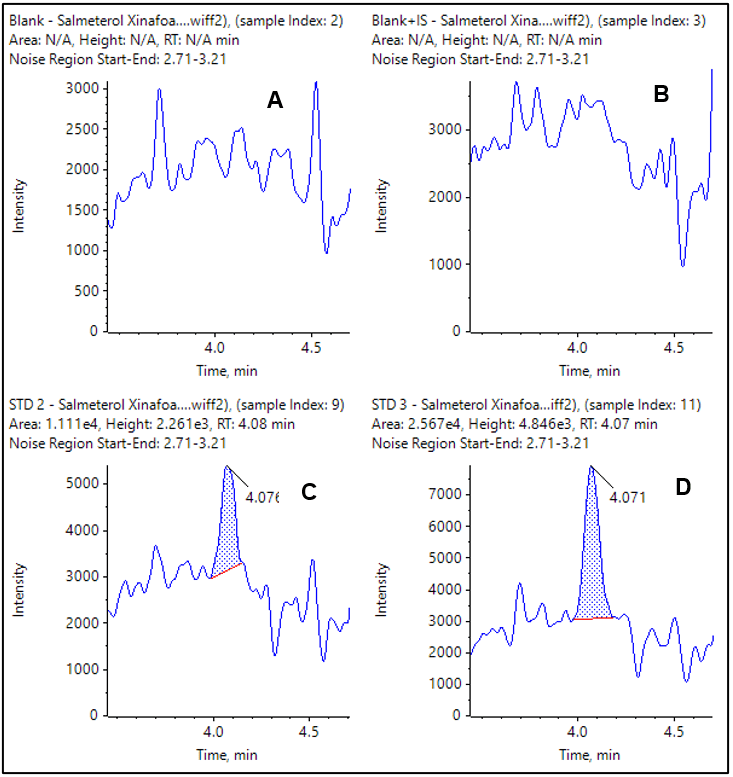 Click to enlarge
Click to enlarge