A sensitive method for the quantification of formoterol in human plasma
Quantification of formoterol using the SCIEX 7500 system with improved front-end technology
Lakshmanan Deenadayalan1 , Sashank Pillai1 , Rahul Baghla2 , Elliott Jones2 and Eshani Nandita2
1SCIEX, India; 2SCIEX, USA
Abstract
This technical note presents a sensitive method for the quantification of the highly potent orally inhaled drug, formoterol, in human plasma. The method employs a simple liquid-liquid extraction sample preparation to extract formoterol from human plasma. Accurate and highly reproducible quantitative performance was achieved with linearity demonstrated at concentrations ranging from 0.05 to 100 pg/mL. The SCIEX 7500 system was used for low-level (0.05 pg/mL) quantification of formoterol in human plasma.
Introduction
As drug discovery and development initiatives concentrate on more effective lower dosage drugs, there is a continual increase in demand for more simple and sensitive bioanalytical methods. The simplest approach to meet these requirements is to utilize a more sensitive mass spectrometer. Bioanalytical scientists have the most flexibility when working with a system that delivers technological advancements that offer sensitivity enhancements across the mass range and in both polarities.1,2
Formoterol is a highly effective, long-acting beta-2-selective adrenoceptor agonist used as a therapeutic to treat pulmonary disorders. The lowest recommended daily dose of formoterol for children 12 years of age and older is 12 µg for oral- and 20 µg for aerosol-based administration. During the drug development pipeline, pharmacokinetic studies could require a dosage as low as 9 µg. Since the concentrations available for analysis are particularly low, the quantification of formoterol in human plasma requires a highly sensitive method. 3,4
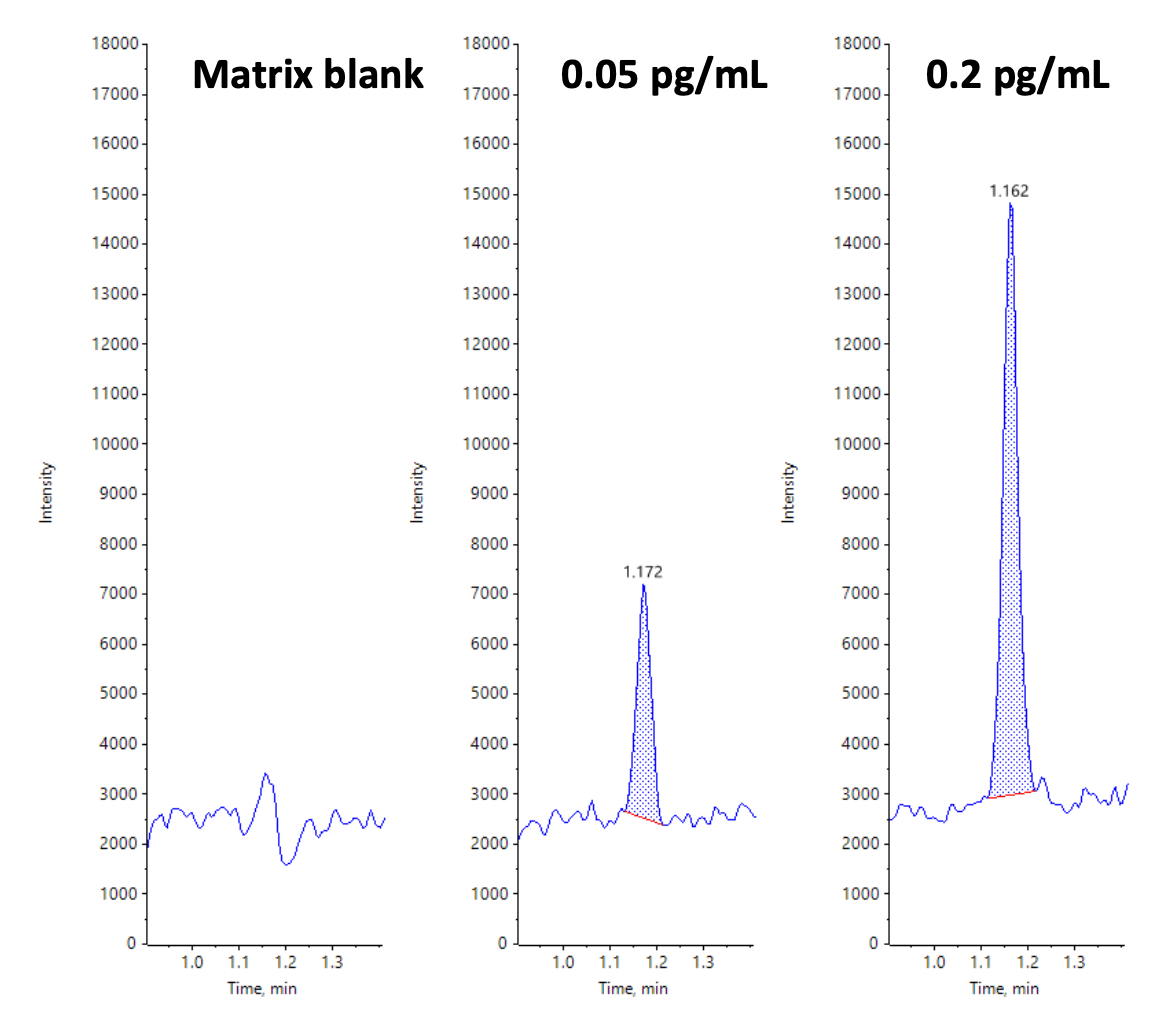
The bioanalytical method described in this technical note uses 300 µL of plasma and a simple extraction method to detect ultra-low levels of formoterol. A lower limit of quantification (LLOQ) of 0.05 pg/mL in human plasma was achieved using the SCIEX 7500 system (Figure 1).
Figure 1. Representative extracted ion chromatograms (XICs) for formoterol in human plasma. The left panel shows results for the matrix blank. The middle and right panels show results for formoterol spiked into human plasma at 0.05 pg/mL (middle) and 0.2 pg/mL (right).
Key features for the analysis of formoterol using the SCIEX 7500 system
- Ultra-low levels of quantification for low-dose, highpotency drug modalities: Achieve an ultra-low level of quantification for formoterol in human plasma on the SCIEX 7500 system equipped with an innovative front-end design
- Minimize sample volume and sample preparation time: Perform sensitive quantification with 300 µL of plasma and simple sample preparation methods by leveraging the high sensitivity of the SCIEX 7500 system
- Effortlessly meet required quantitative criteria: Achieve accurate and highly reproducible quantitative performance at low concentration levels
- Streamlined data management: Data acquisition and processing are simplified with SCIEX OS software, a 21 CFR Part 11-compliant platform
Methods
Spiked sample preparation: Formoterol fumarate was spiked into 300 µL of human plasma at concentrations ranging from 0.05 to 100 pg/mL and 50 pg of internal standard was added. Samples were extracted using liquid-liquid extraction with 2.5 mL of tert-butyl methyl ether and 200 µL of 0.1% (v/v) ammonium hydroxide solution in water. The samples were vortexed for 3 mins and centrifuged at 3901 RCF for 5 mins. The supernatant was collected and dried under nitrogen at 40°C. Dried samples were reconstituted using 150 µL of 80:20 (v/v), methanol/10mM ammonium acetate in water.
Chromatography: An ExionLC system with a Phenomenex Luna Omega Polar C18 column (2.1 x 100 mm, 3 µm, 100 Å) was used for chromatographic separation at a flow rate of 0.6 mL/min. The column was operated at 40°C. Mobile phase A was 0.1% (v/v) acetic acid in water and mobile phase B was methanol. Table 1 summarizes the LC gradient conditions used.
Table 1. LC gradient.
Mass spectrometry: Samples were analyzed using the SCIEX 7500 system equipped with the OptiFlow Pro ion source and the system was controlled by SCIEX OS software. The optimized MS parameters are listed in Table 2.
Data processing: Data processing was performed using SCIEX OS software, version 3.0. Peaks were automatically integrated using the MQ4 algorithm with a weighting of 1/x2 .
Table 2. Optimized MS parameters.
Quantitative performance
A calibration curve was analyzed for concentrations ranging from 0.05 to 100 pg/mL. To evaluate reproducibility, each concentration of formoterol was analyzed in triplicate.
An LLOQ of 0.05 pg/mL was achieved for formoterol in human plasma. No interferences were observed in the matrix blank (Figure 1). Linearity was observed across concentrations ranging from 0.05 to 100 pg/mL with a regression coefficient (r2 ) >0.99 and a linear dynamic range (LDR) of 3.2 orders of magnitude (Figure 2). No carryover was observed for the blank injection following analysis of the highest concentration tested.
Analytical performance was evaluated based on the requirement that the accuracy of the calculated mean should be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean of the concentration should be below 20% at the LLOQ and below 15% at all higher concentrations.5
For this assay, accuracy was within ±8% of the nominal concentration and %CV was <11% for formoterol in human plasma (Table 3). Calculated percent accuracy and %CV values were within the acceptance criteria at each concentration level (Table 3).
Table 3. Summary of the quantitative performance. Reproducibility and accuracy results were determined from the calibration curve across 3 replicates.
Compliant-ready SCIEX OS software
To meet the regulation outlined in 21 CFR Part 11, SCIEX OS software is designed as a closed system, and includes the requirement for the records and signatures to be stored electronically. SCIEX OS software can open raw data files from any visible storage location, which enables the flexibility to work within a closed network using designated processing workstations. Figure 3 illustrates 3 types of controls that are required for 21 CFR Part 11 compliance. The workflow presented here is fully compliant with these guidelines, as SCIEX provides 1) technical controls over hardware and software configuration, 2) network security and secure operating systems and policies and 3) procedures and user training (Figure 3)
Figure 3. Controls required for 21 CFR Part 11 compliance.
Content here
Conclusion
- Ultra-low quantification levels were achieved for formoterol in human plasma using a simple liquid-liquid extraction sample preparation
- The method demonstrated accurate and highly reproducible quantitative performance at all concentration levels
- The assay exhibited the ability to routinely quantify ultra-low levels of formoterol in an 8-min runtime, enabling bioanalytical labs to deliver high-quality data with excellent throughput
- A single platform for streamlined data acquisition, processing and management with SCIEX OS software was presented
References
- Enabling new levels of quantification. SCIEX technical note, RUO-MKT-02-11886-A.
- Achieve next level sensitivity for the evolution of routine bioanalysis. SCIEX technical note, RUO-MKT-02-14859-A
- Mascher DG. et al. (2006) Ultra-sensitive determination of Formoterol in human serum by high-performance liquid chromatography and electrospray tandem mass spectrometry. Journal of Chromatography B, 830(1):25-34.
- Drugs and supplements, Formoterol (Inhalation Route), Mayo Clinic, Nov 2022.
- Bioanalytical Method Validation, May 2018.
 Click to enlarge
Click to enlarge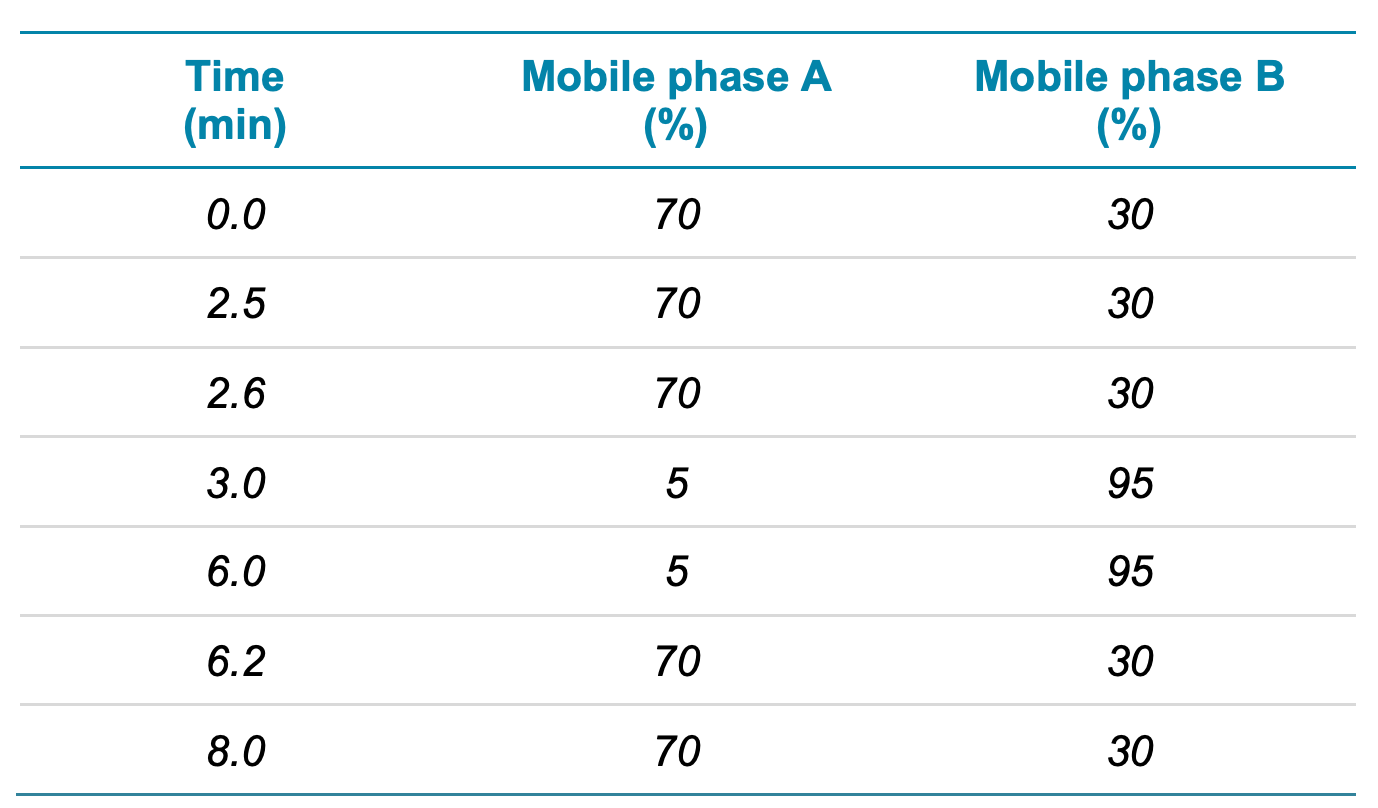 Click to enlarge
Click to enlarge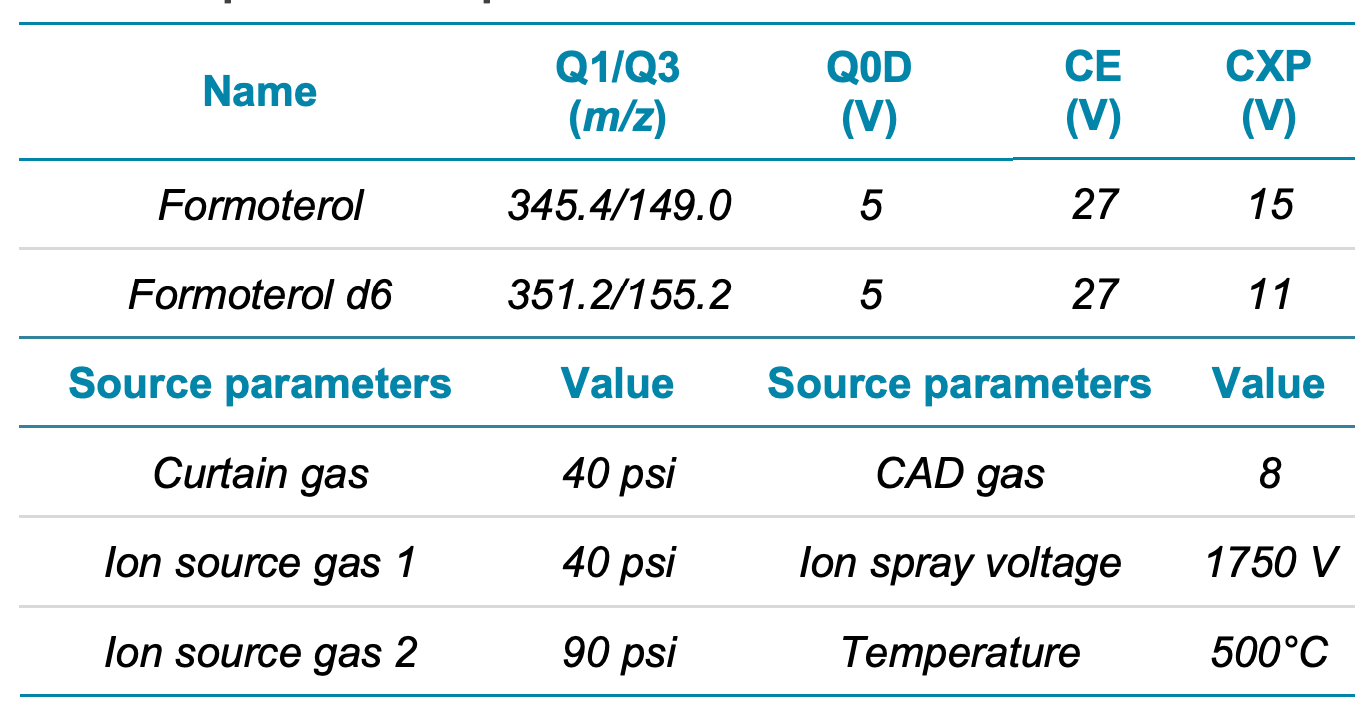 Click to enlarge
Click to enlarge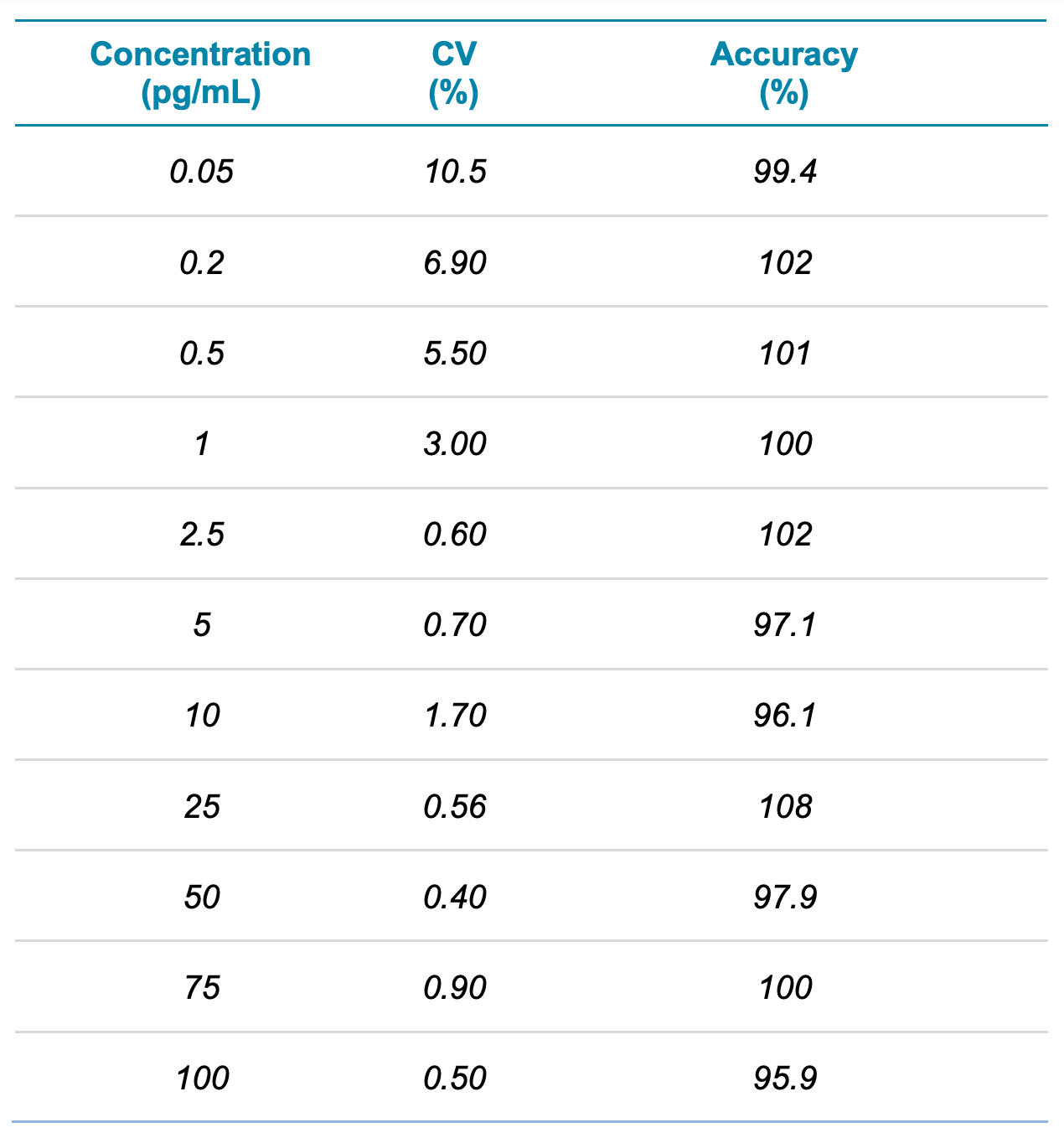 Click to enlarge
Click to enlarge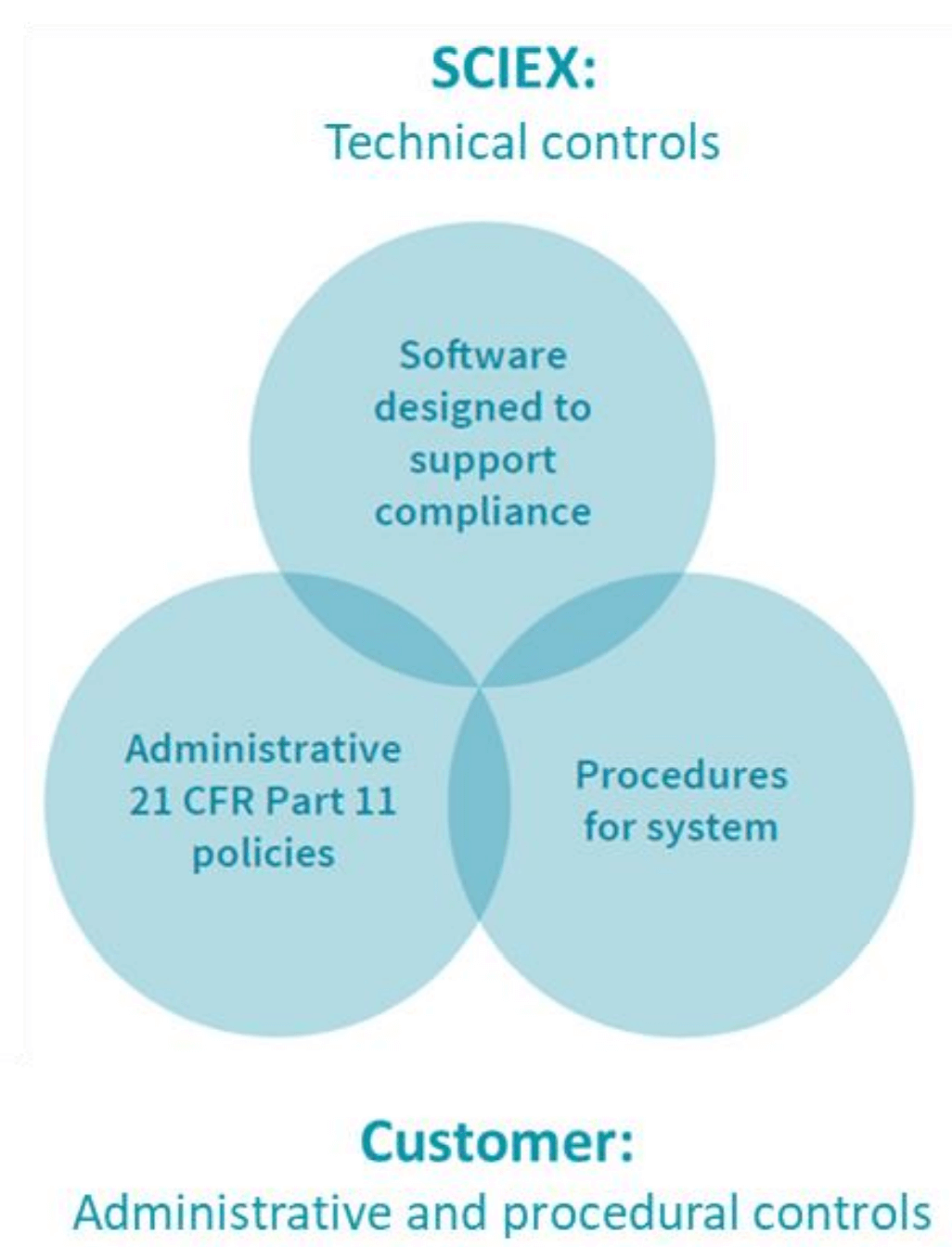 Click to enlarge
Click to enlarge