Overview
The development of viral vector vaccines and therapeutics means overcoming many challenges.
Intuitive, innovative and informative analytical solutions for viral-vector characterization help ensure safe and effective drugs and let you stay focused.
Work with an evolving partner to realize the full potential of using viral vectors—such as adenoviruses (AVs), adeno-associated viruses (AAVs) and lentiviruses (LVs)—and virus-like particles as delivery vehicles for nucleic acid-based gene therapies and vaccines.
Workflow
Genome integrity and protein profiling
Viral vector characterization for vaccine and therapeutic drug development includes assessing multiple critical quality attributes (CQAs). This can be a cumbersome task, especially when different serotypes require assay adjustments.
Save time by characterizing multiple CQAs with serotype-independent workflows on a single platform. The ability to determine viral protein profiles, genome integrity, nucleic acid impurities, titer and full and empty ratios with high sensitivity on a user-friendly system lets you move your viral vector programs forward with confidence.
-
Simplify vector analysis with high-definition, serotype-independent workflows
-
Assess multiple CQAs with high-quality data on a single platform
-
Determine capsid protein profiles, genome integrity, impurities and titer
-
Cover your compliance needs through compatibility with the Empower Chromatography Data System (CDS)
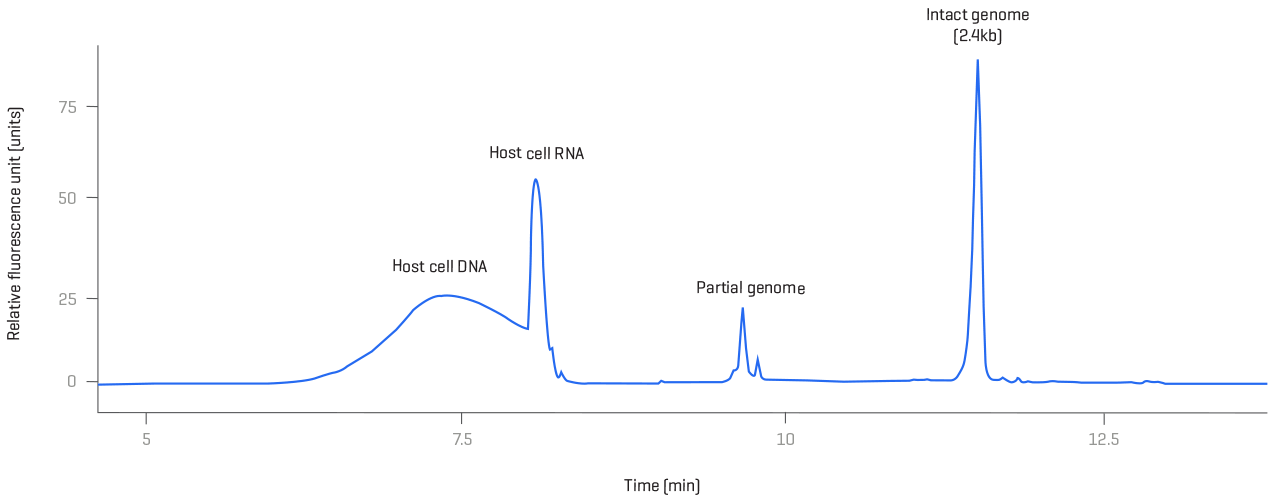
Genome integrity and protein profiling
Solution
- Assessment of multiple CQAs
- Faster method development and larger sample sets
Featured resources
Learn how to break through the boundaries of critical quality attribute (CQA) determination of adeno-associated viruses (AAVs) using a single platform. Leverage high-resolution data to push your viral vector development forward with platform methods.
Reach new milestones by assessing adeno-associated virus (AAV) critical quality attributes (CQAs) on an intuitive platform that provides sensitive, high-quality data for multiple viral-vector quality attributes.
Genome integrity and protein profiling
Solution
Featured resources
Set new frontiers for protein profiling and protein purity assessment of AAVs with an intuitive solution that provides accurate and sensitive and informative data.
Workflow
Viral protein characterization
Viral capsid proteins can impact uptake by cells and therefore directly impact their effectiveness. Thorough protein characterization helps ensure the quality of your viral vectors.
Assess capsid protein integrity through high-quality data coupled with powerful intact mass deconvolution and elucidate the identities and positions of challenging post-translational modifications (PTMs) that can impact safety and efficacy.
Move beyond boundaries of low sample amounts with highly sensitive workflows.
-
Identify PTMs and their locations, including sulfations, phosphorylations, glycosylations and amino acid isomers
-
Obtain high sensitivities despite limited sample quantities
-
Fully understand your intact proteins and PTMs with flexible data-processing software
-
Get started quickly and confidently with intuitive control software
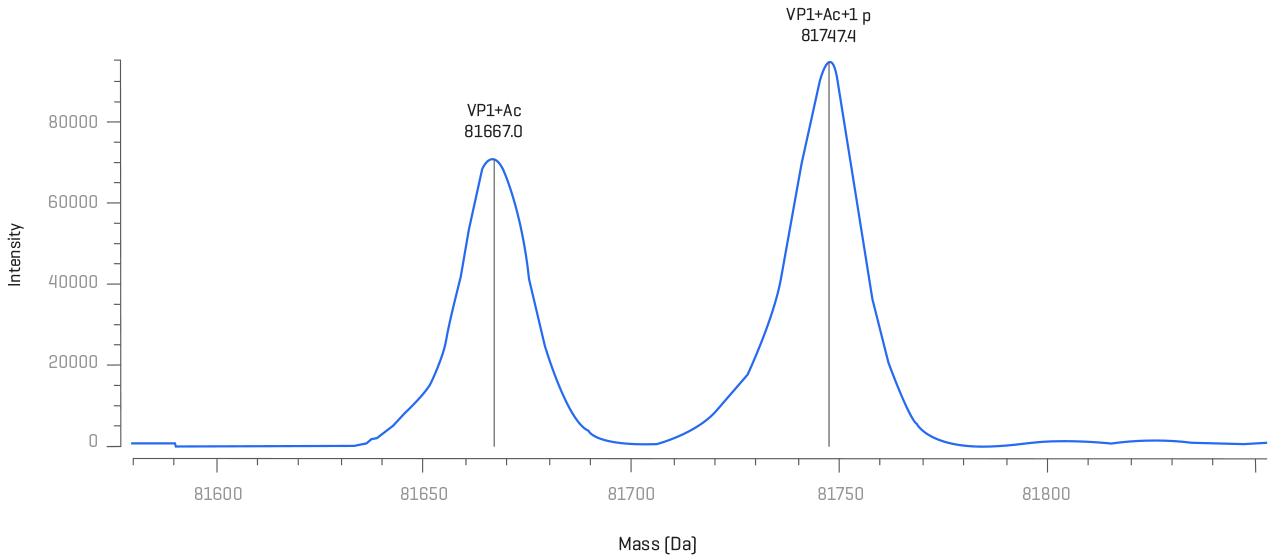
Viral protein characterization
Solution
- Differentiation of isomers and identification of challenging PTMs
- Highly flexible LC coupling and other workflow options
Featured resource
Uncover a sensitive and easy-to-adopt solution that enables confident identification of low abundance proteins and post-translational modifications (PTMs), including structurally similar isomers that can impact vector infectivity and efficacy.
Viral protein characterization
Solution
- Intact VP protein characterization and peptide mapping
- Intuitive operation
Workflow
Host cell protein analysis
The changing landscape of packaging cell lines and the need to combat future pandemics with unprecedented response times require highly adaptable solutions for detecting process-related impurities in viral vector products.
High-quality data from fast scanning solutions provide excellent coverage and depth to identify thousands of low abundance residual host cell proteins without the need for lengthy assay development.
Enable your team to ensure product safety and keep processes moving with confidence.
-
Avoid missing critical impurities using data-independent acquisition (DIA)
-
Be empowered by high sensitivity despite limited sample amounts
-
Identify thousands of impurities with the highest data quality while reducing run times
-
Get started quickly and confidently with intuitive control software
Host cell protein analysis
Solution
- Untargeted identification of process-related impurities
- Highly sensitive quantitation
Featured resource
Dr. Ejvind Mørtz (Alphalyse) is changing the paradigm of host cell protein analysis with highly flexible, sensitive and reliable MS solutions. Discover workflows that offer more detailed information on impurities in viral vector products.
All resources
Learn how to break through the boundaries of critical quality attribute (CQA) determination of adeno-associated viruses (AAVs) using a single platform. Leverage high-resolution data to push your viral vector development forward with platform methods.
Reach new milestones by assessing adeno-associated virus (AAV) critical quality attributes (CQAs) on an intuitive platform that provides sensitive, high-quality data for multiple viral-vector quality attributes.
Set new frontiers for protein profiling and protein purity assessment of AAVs with an intuitive solution that provides accurate and sensitive and informative data.
Take control of the quality of your lentiviral vectors with high performance solutions. Learn how to characterize proteins, determine protein profiles, and enhance your understanding of process change.
Uncover a sensitive and easy-to-adopt solution that enables confident identification of low abundance proteins and post-translational modifications (PTMs), including structurally similar isomers that can impact vector infectivity and efficacy.
Discover an innovative way to achieve protein deconvolution with 3-dimensional data visualization. See more and miss fewer relevant features of your sample.
Dr. Ejvind Mørtz (Alphalyse) is changing the paradigm of host cell protein analysis with highly flexible, sensitive and reliable MS solutions. Discover workflows that offer more detailed information on impurities in viral vector products.