iMethod Application for Ethyl Sulfate and Ethyl Glucuronide
For research use only. Not for use in diagnostic procedures.
This method analyzes ethyl glucuronide (EtG) and ethyl sulfate (EtS) in human urine. Samples are prepared by dilution, centrifugation, and injection.
Results show that both EtG and EtS can be accurately quantified in this procedure, with percent accuracies ranging from 99.2 to 104% for both EtG and EtS. These results show that this method is rigorous, reliable, and robust for the quantification of EtG and EtS in human urine.
For system requirements and example results, read the flyer:iMethod™ Application for EtG and EtS.
Status: Available

Just download a method, add your sample, and you're ready to go
iMethod™ Applications for forensic toxicology provide instant LC/MS/MS methods, enabling you to generate accurate and precise results faster. Simply download a lab-proven method from the web or a CD into Cliquid® Software and run your sample. All the instrument parameters are downloaded upon installation. Everything you need to start running a test immediately is included: sample preparation protocolss, optimized MS and LC parameters, integration parameters, reporting templates, and documentation.
Rapid analysis of ethyl sulfate and ethyl glucuronide in human urine
Ethyl glucuronide (EtG) is a direct metabolite of ethanol. The presence of EtG in urine can be used to detect ethanol up to 80 hours after initial ingestion. EtG testing is not subject to adulteration, fermentation, or cross reactivity. Ethyl sulfate (EtS) has also been identified as a second specific metabolite of ethanol. Measuring both EtG and EtS provides greater sensitivity and accuracy.
This iMethod™ Application uses a simple sample preparation procedure from human urine to detect and quantify EtG and EtS. Internal standards are added to the urine and then the sample is diluted with water. This method was optimized using the following specific supplies and equipment: (1) Phenomenex Synergi 4 µm Hydro-RP analytical HPLC column (100 x 4.6 mm), (2) a Shimadzu Prominence LC system, and (3) an API 3200™, 3200 QTRAP®, API 4000™ or 4000 QTRAP® LC/MS/MS System.
The method performance was verified in multiple labs using fortified control samples. A sample chromatogram of the EtG and EtS MRMs and the accuracy and precision results obtained in the method verification are shown in Figure 1 and Table 1.
Figure 1. Sample chromatogram of MRMs of EtG and EtS detected at 100 ng/mL in a human urine sample.
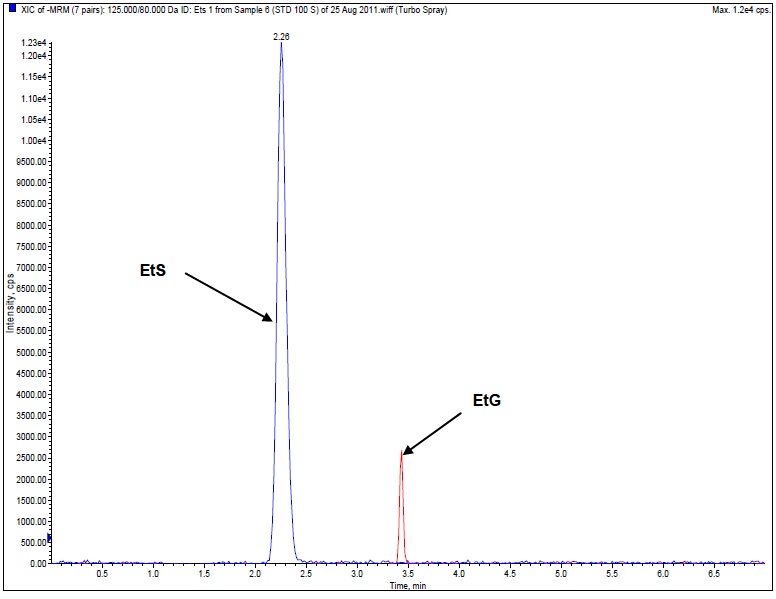
Table 1. Outline of results obtained for EtG and EtS.
| Instrument | %CV | % Accuracy 30 ng/mL | %CV | % Accuracy 250 ng/mL | %CV | % Accuracy 3000 ng/mL | Calibration range (ng/mL) | Correlation coefficient |
|---|---|---|---|---|---|---|---|---|
| EtG using 3200 QTRAP® System | 4.78 | 100 | 3.65 | 103 | 2.28 | 104 | 10 to 4000 | 0.9997 |
| EtS using 3200 QTRAP® System | 2.11 | 104 | 1.27 | 104 | 0.97 | 101 | 10 to 4000 | 1.0000 |
| EtG using 4000 QTRAP® System | 5.80 | 101 | 4.84 | 99.9 | 3.90 | 101 | 10 to 4000 | 0.9990 |
| EtS using 4000 QTRAP® System | 4.82 | 101 | 4.36 | 101 | 3.71 | 99.2 | 10 to 4000 | 0.9993 |
The list of available iMethod™ Applications located on the iMethod™ Store is continually updated with the newest user-contributed and SCIEX lab-proven methods, so you can take immediate advantage of the latest advances. All iMethod™ Applications are available for individual purchase or as part of one of our complete starter packs for forensic toxicology.
Reduce development time
| Activity | Using an iMethod™ Application | Traditional Method Development |
|---|---|---|
| Complete Test Protocol Documentation | Included | Weeks–Months |
| Sample Prep Techniques | Included, with examples and references | Weeks–Months |
| Optimized MS/MS Acquisition Parameters | Included | Weeks–Months |
| LC Instrument Parameters with Retention Times | Included | Days–Weeks |
| Integration (Quantitation) Parameters | Included | Days |
| Reporting Templates | Included | Days |
| LC/MS/MS Library Spectra | May be included. Stand-alone libraries are also available. | Weeks–Months |
| Staff Training – Software | Simple Cliquid® Software interface, no additional training | Traditional MS Software and review procedures – Days |
| Method Validation | Weeks. Validation templates and support available | Weeks–Months |
| Complete Test Development and Deployment | Weeks | 6–12 months |
 Request a Quote
Request a Quote
 CONTACT SUPPORT
CONTACT SUPPORT