연구 목적으로만 사용할 수 있습니다. 진단 절차에는 사용할 수 없습니다.
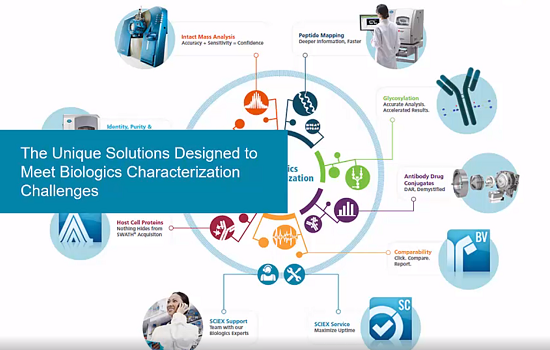
특성 분석 및 동등성에 대한 바이오치료제 데이터 처리를 간소화시키면 생산성이 획기적으로 향상됩니다. BioPharmaView 소프트웨어는 특성 분석과 동등성 연구를 촉진시키고 보고서 작성을 간소화시키며, 이를 통해 보다 나은 결정을 신속하게 내릴 수 있습니다.
TripleTOF® 또는 X500B QTOF Accurate Mass 시스템에서 실행된 워크플로우로부터 감소되거나 감소되지 않은 상태의 인택트 단백질이나 프로테아제 소화 생성물을 분석하면 결과를 종합적으로 볼 수 있습니다. 이제 이러한 성능을 활용하여 데이터 독립적 SWATH® Acquisition 분석을 실행할 수 있습니다. 대규모 데이터 집합에도 BioPharmaView 소프트웨어를 통해 사이트 간 또는 로트 간 차이점을 신속하게 동정할 수 있습니다. DAR, PTM 비율 및 이황화물 결합 위치와 같은 소프트웨어의 핵심 속성 자동 계산 성능을 통해 데이터에서 원하는 답을 신속하게 찾을 수 있습니다.


BioPharmaView Software 3.0 is Here!
Simplify your Product Characterization and Multiple Attribute Methodology Assay Development with this Powerful New Software. Register your internet for a product demo and learn how to upgrade your software!
Powerful
Boost your biotherapeutic characterization analyses with automated data processing tools that allow you to: Assess intact mass, subunit, and peptide mapping data quickly Easily identify product differences from site-to-site and lot-to-lot Perform a streamlined workflow for MAM to assess PQAs Directly monitor known product impurities or host cell protein contaminants Detect and flag new, unspecified impurities found in a sample
Intuitive
The software is designed from the ground up with both experts and non-experts in mind, so you can focus on delivering results quickly. The BioPharmaView Software is complete with tools to accelerate your time to results: Process in-depth biologic characterization data at the intact, subunit, or peptide level Perform a streamlined workflow for MAM to monitor PQAs and detect potential product impurities
Efficient
Spend more time understanding your data and less time processing samples. With BioPharmaView Software you can: Crunch numbers easily with batch processing functions and automated ratio calculations for both post-translational modifications as well as drug-to-antibody (DAR) analysis Customize calculations and comparison tables for a holistic view of your attribute data
A Complete Workflow for Multiple Attribute Methodology (MAM)
Develop and implement a Multiple Attribute Methodology (MAM) workflow with the new capabilities of BioPharmaView Software. This streamlined methodology can be used to replace, or complement, several conventional process development methods in a single simplified analysis.
BioPharmaView Software makes moving to a powerful high-resolution, accurate mass LC-MS based MAM assay easier with the all-in-one software to:
- Define, track, and quantify product quality attributes (PQAs)
- Monitor known potential impurities or host cell protein contaminants
- Perform unspecified impurity testing with new peak detection algorithms

Get Accurate Intact Protein Deconvolution in Seconds
Leverage the power of BioPharmaView Software tools for fast and accurate intact mass analysis and when you compare biologic product characteristics.
The easy one-click batch processing feature and automated ratio calculations for post translational modifications—helps you to compare multiple products quickly and efficiently.
The multi-pane view in the main window allows you to see processed and raw data from multiple samples side by side so that you can be confident about your comparability conclusions.

Antibody Drug Conjugate Analysis Made Easy
Intact analysis of antibody-drug conjugates (ADCs) can be challenging because of the size and complexity of the molecules. With BioPharmaView Software there’s no need to worry about your ADCs as the software:
- Automates drug-antibody ratio (DAR) calculation and visualization
- Presents a simplified—yet highly accurate view of protein deconvolution
You can now compare ADCs easily as you can see drug load and DAR across multiple samples. You can always be clear on just what you’ve got—and how much.

Achieve Rapid Answers for Peptide Mapping Analyses
Go from peptide mapping data to answers in four easy steps with the BioPharmaView Software.
- Get a quick look at protein sequence coverage from your peptide mapping data
- Quickly confirm each peptide using a comprehensive list of identified peptides that are directly linked to high-resolution TOF-MS and annotated MS/MS data

Capture Low Abundance Components with SWATH® Acquisition
Proprietary SWATH® Acquisition for peptide mapping provides comprehensive data collection and eliminates the need for IDA criteria set-up and traditional method development.
Acquire high-resolution MS/MS for all precursor ions including all low abundance peptides and post-translational modifications (PTMs) with SWATH Acquisition. You can be confident that you’re truly collecting comprehensive and unbiased data and haven’t missed information dependent on peptide map workflows.
A standard, generic SWATH method can also be used in almost every biotherapeutic peptide mapping analysis further simplifying your workflow setup and reducing your time to results.

Automatically Map Disulfide Bonds
Let the software quickly and accurately map disulfide bond locations to simplify your data analysis. The built-in algorithms in BioPharmaView Software quickly:
- Defines the disulfide bond locations
- Presenting high-resolution, annotated MS/MS spectra including multiple charge state identification for confirmation

On Demand Webinar
Watch the full webinar to get insight to a Streamlined Approach for Biotherapeutic Characterization.
동영상 길이
Resources
Software |
|
|
|
|
|---|---|---|---|---|
Name | File Size | Release Date | Release Notes | Download |
108 MB | 12.19.19 | |||
| 12.19.19 |
| ||
133.9 MB | 10.01.19 | |||
| 10.01.19 |
| ||
| 08.19.19 |
| ||
87.6 MB | 08.19.19 | |||
133 MB | 11.30.18 | |||
| 11.30.18 |
| ||
| 05.10.18 | |||
| 05.10.18 |
| ||