Separation of ephedrine and pseudoephedrine isomers using SCIEX SelexION® Differential Mobility Technology
Yang Zong1, Cheng Haiyan1, Li Lijun1, and Jin Wenhai1
1SCIEX, China
Introduction
Ephedrine and pseudoephedrine are major alkaloids extracted from ephedra. These two compounds are cis-trans-isomers due to different positions of the hydroxyl group, and thus have different pharmacological mechanisms. Ephedrine is a stimulant that is widely used as a weight-loss drug in healthcare products. Its hydrochloride can help alleviate bronchial asthma. It is also an important raw material for synthesising methamphetamine, a banned drug. Ephedrine is addictive in nature and is banned by law, but, pseudoephedrine is not addictive and is commonly used in cold medicine as nasal decongestant. Their differences in pharmacological mechanisms have gained much attention, particularly in the field of pharmacokinetic studies in the human body.
Challenges in LC-MS Analysis
Both compounds have weak retention on analytical column and usually elute together with matrices which cause ion suppression. It is not easy to achieve baseline separation even in UHPLC conditions. Both compounds have identical mass spectra, identical parent ions and fragment ions. There are no unique fragments to distinguish ephedrine/ pseudoephedrine.
Figure 1: Mix standard of ephedrine and pseudoephedrine. A is the BPC plot; B: XIC of parent ion of ephedrine, C: XIC of parent ion of pseudoephedrine with XIC at 166.1232 +/-0.01 Da.
Key advantages of method
- Ephedrine and pseudoephedrine were separated successfully with SelexION Technology
- Excellent sensitivity was achieved when SelexION Device was used with the MRMHR Workflow on the TripleTOF 5600 System
- Short chromatographic runtime of 4min and ability to minimise matrix interference with the help of SelexION Technology
- Simple and efficient sample extraction method which took 10min to prepare 10 plasma and urine samples
- Wide dynamic range between 0.1 and 1000 ng/mL in spiked matrices, with a good linear correlation, r ≥0.995
- In low (1ng/mL), medium (5ng/mL) and high (20ng/mL) concentrations, the method had recovery efficiency of between 88.38 and 100.80 % and the excellent repeatability of less than 5% with n=6
- This method is quantitative; ephedrine/pseudoephedrine were measured accurately in plasma samples and urine samples
- This method could serve as a reference method for forensics analysis of narcotic drugs
Methods
Sample preparation: Sample extraction is fast and simple which takes only 10 minutes for 10 plasma samples and urine samples including of analysis time on the instrument. The extraction flowchart is shown in Figure 3.
LC-MS conditions: Separation was performed on the SCIEX ExionLC™ AC System using a Phenomenex C18, 2.6µm, 3.0 × 50mm and standard reverse phase mobile phase and gradient elution was used. Autosampler settings were as follows: injection volume was 2 µL, temperature of 15 °C, needle depth was 52mm and injection speed was 5 µL/min.
Mass spectrometry: The TripleTOF® 5600 System equipped with the SelexION® Device was used for data acquisition, using the MRMHR Workflow. Electrospray Ionization (ESI) was conducted in positive ion mode. Operating parameters for differential mobility separation are outlined in Table 1.
Table 1. DMS parameters. Optimized parameters for SelexION device are shown.
Figure 2: Fast and simple sample extraction procedures. |
Studying the molecule fragmentation
Ephedrine and pseudoephedrine are cis-trans-isomers due to difference in the position of hydroxyl group (Figure 3). The positional bond energy of the hydroxyl group is -135.32 kJ/mol (Gibbs Energy), meaning that it breaks easily. At declustering potential (DP) of 10V, it can break off and resulting in-source collision-induced dissociation (CID) as shown in Figure 4 (top).
MS/MS fragmentation was performed to assess the fragmentation patterns for both molecules, as expected there were no unique fragment ions, making separation using differential mobility critical (Figure 4). As full scan data is collected during MRMHR workflow, XICs of the most sensitive and specific fragment ions can be done post-acquisition.
Figure 3: Structure of ephedrine and pseudoephedrine.
Figure 4. Spectra of ephedrine. (Top) TOF MS of ephedrine showing in-source fragmentation. (Bottom) TOF MS/MS of ephedrine with the assignment of fragments.
Optimization of DMS separation conditions
The experiment found that ephedrine and pseudoephedrine is best separated when isopropanol was used as a modifier as shown in Figure 5 and Figure 6.
In order to confirm that the use of specific compensation voltages will provide clean signal for each of the isomers, LC-MS experiments were performed where one isomer was injected on column and both CoVs were monitored. If the separation was complete, no signal should be observed at the CoV determined for the other isomer.
When injecting the ephedrine standard solution, a good signal is observed for ephedrine (at the ephedrine CoV of -44V) but no signal is seen for at the pseudoephedrine CoV of -41.8V (Figure 7). Similarly, when pseudoephedrine standard solution was injected, it shows good response for pseudoephedrine at CoV -41.8V but no signal is seen at the CoV for ephedrine of -44V (Figure 8). This highlights that nearly complete separation between ephedrine and pseudoephedrine can be obtained using the SelexION Technology using a short LC runtime.
Figure 5: COV ramp graph for ephedrine/ pseudoephedrine. Infusion of the two isomers while ramping the CoV voltage at a fixed separation voltage of 3500V shows unique COV values for ephedrine (-44V) and pseudoephedrine (-41.8V).
Figure 6: Altering separation with chemical modifiers. Different modifiers were explored to determine which would provide the best separation of these two isomers, with 2-propanol providing the best separation using SelexION device.
Figure 7: Clear separation of ephedrine. LC-MS was performed using a short run time on Ephedrine only and the XIC of the most intense fragment for Ephedrine/Pseudoephedrine was monitored. At the determined CoV of -44V for Ephedrine (bottom), good signal is seen. At the CoV of -41.8V for Pseudoephedrine (middle), no signal is observed showing very clean separation.
Figure 8: Clear separation of pseudoephedrine. In a similar experiment to Figure 7, LC-MS was performed injecting Pseudoephedrine only and good signal is seen at the Pseudoephedrine CoV (-41.8 V, middle) and no signal is observed at the Ephedrine CoV (-44V, bottom).
Linearity, recovery, repeatability & matrix effects
Ephedrine/ pseudoephedrine were spiked in plasma and urine matrices with concentration ranging from 0.1 to 1000ng/mL. Both matrices show good linear correlation with r greater than 0.995 as shown in Figure 9.
Extraction recovery was tested at low (1 ng/mL), medium (5 ng/mL) and high (20 ng/mL) level shown in Table 2. The recovery was found to be 88.38% - 108.8% in plasma and urine. Excellent reproducibility was achieved with CV within 5% with N=6.
To highlight the impact of DMS on reducing matrix interferences, LC-MS runs with the isomers in matrix (plasma and urine) were run collecting TOF MS data, with and without DMS on. Post-acquisition extractions of the parent ion m/z (166.123 m/z) with and without the DMS were compared. In the plasma matrix, the S/N ratio on ephedrine improves from 30 (DMS off) to 76.6 (DMS on, -44V) as seen in Figure 10 (top). In the urine sample, the chromatogram shows significant baseline noise due to severe matrix interferences when DMS was turned off (SN: 30.5). The S/N ratio improves by two times for ephedrine when DMS was turned on (SN: 62.7) as shown in Figure 10 (bottom). The SelexION Technology has proven to reduce matrix interference and helps to improve the S/N ratio which resulted in better sensitivity in this analysis method, even when only analysing the parent ion signal.
Figure 9: Good linearity in matrix. Concentration curves were analyzed in two different matrices, plasma and urine. Good linearity was obtained for both ephedrine (blue) and pseudoephedrine (pink).
Table 2. Recovery and repeatability tests for low, mid & high levels.
Figure 10: Reduction of background interferences using SelexION Device. DMS helps to reduce background noise and specific interferences in both matrices studied (plasma – top, urine – bottom). Here, the CoV value of -44V was used for specific detection of ephedrine.
Analysing ephedrine and pseudoephedrine in real samples
The concentration of ephedrine and pseudoephedrine was studied in human plasma and urine. Results show that both isomers were highest in matrix 2 hours after administration. Higher concentrations were observed in plasma vs in urine samples. Metabolism of ephedrine and pseudoephedrine behaves similarly and will complete after 15 hours (Figure 11).
Figure 11: Metabolism of ephedrine and pseudoephedrine. The concentration plot to show metabolism of ephedrine/ pseudoephedrine in plasma and urine samples, similar behaviors were seen for both isomers.
Conclusions
Here, the SelexION Technology in combination with the MRMHR workflow method on the TripleTOF 5600 System was used to provide accurate quantitation of two isomers, ephedrine and pseudoephedrine, in plasma and urine samples. With differential mobility separation and the use of chemical modifiers, good separation was achieved between the 2 enantiomers using the SelexION device. Because of this, very simple, efficient sample extraction could be used. Short LC-MS run times (4 minutes) could also be used because of the efficient separation achieved.
This analysis method provided good linearity over a wide concentration range (ranging from 0.1 to 1000ng/mL), with r ≥ 0.995. Good extraction recovery was achieved, with recoveries of 88.38 - 100.80% the 3 concentration levels tests, with excellent CV of less than 5%.
Finally, a metabolism study was performed and both compounds show highest concentration in plasma and urine 2hrs after administration. The concentration level of both compounds is higher in plasma than in urine. The metabolism will be almost complete in 15 hrs for both compounds. This analysis method could provide a solution fast and accurate quantitation of ephedrine and pseudoephedrine in plasma and urine samples in forensic lab for narcotics analysis.
References
- S Kreppenhofer, B Casetta, MJY Jarvis et al; (2012) Analysis of estrogens in plasma sample by LC–MS/MS with a reduced sample preparation afforded by the use of SelexION ion mobility technology. Clinical Biochemistry 45, 13 – 14.
- Schneider et al, (2010) Chemical Effects in the Separation Process of a Differential Mobility / Mass Spectrometer System. Analytical Chemistry 82, 1867 – 1880.
- N Gray, J Heaton et al, (2013) Comparison of reversed – phase and hydrophilic interaction liquid chromatography for the quantification ofephedrines using medium – resolution accurate mass spectrometry. Journal of Chromatography A, 1289, 37 - 46.

 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge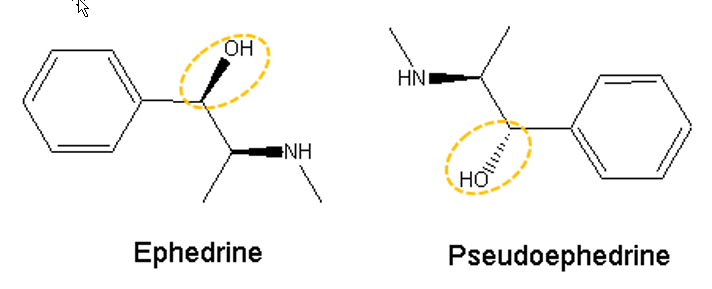 Click to enlarge
Click to enlarge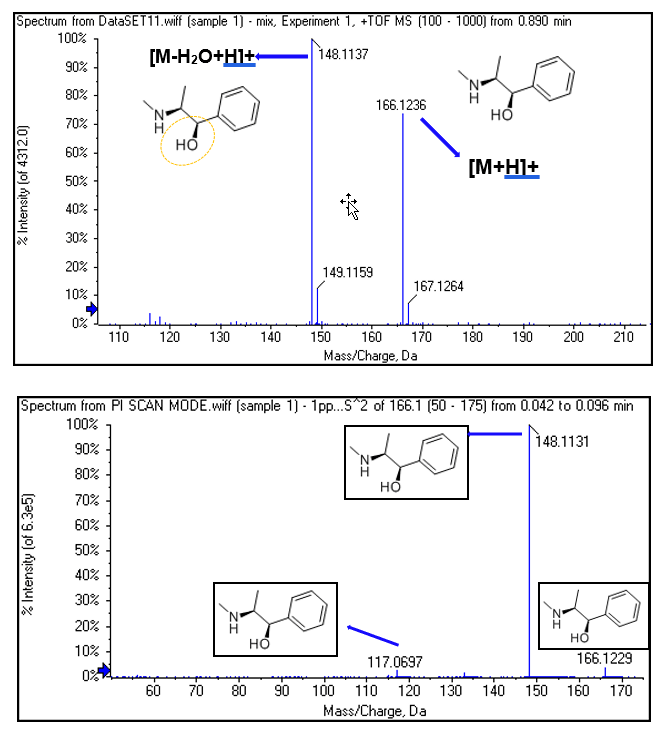 Click to enlarge
Click to enlarge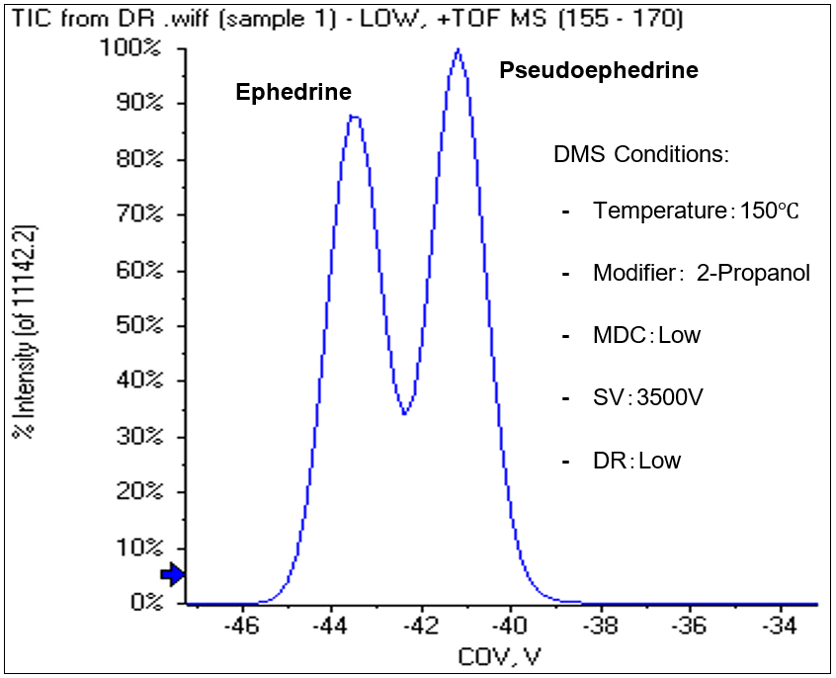 Click to enlarge
Click to enlarge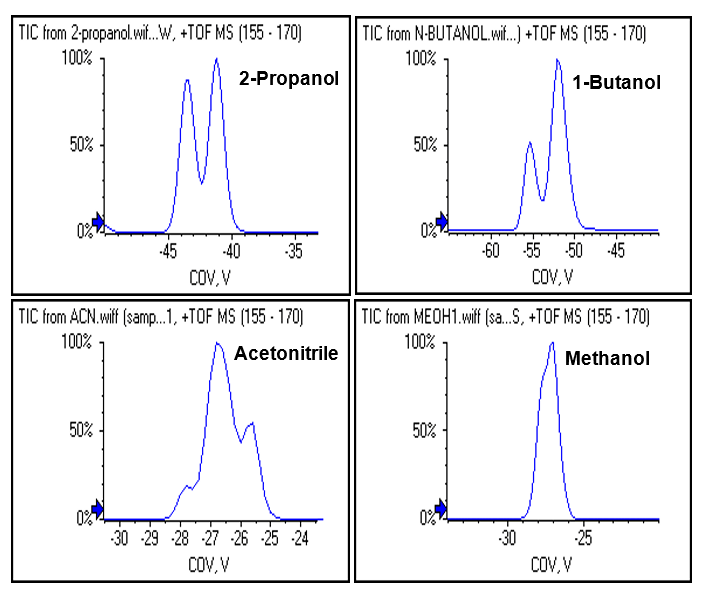 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge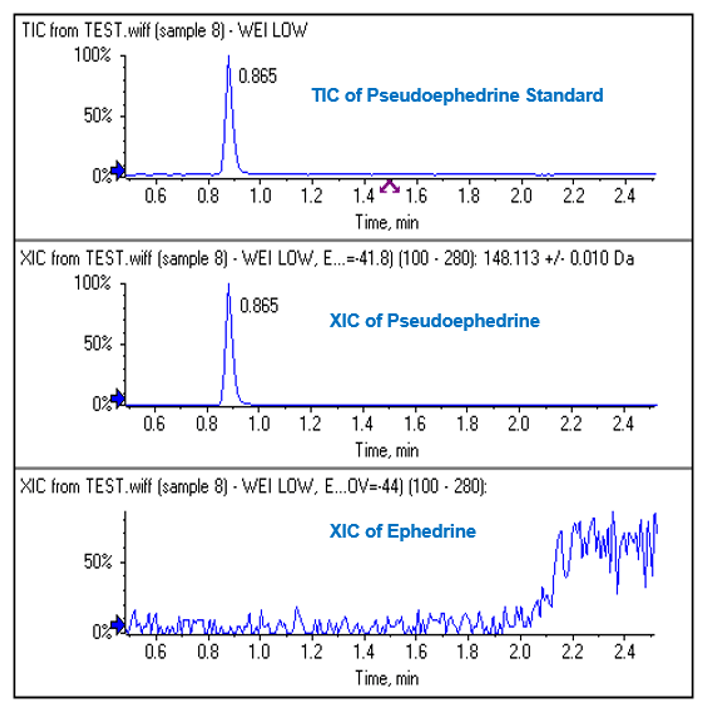 Click to enlarge
Click to enlarge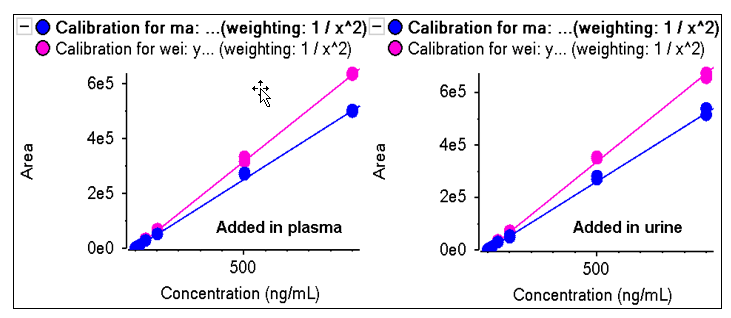 Click to enlarge
Click to enlarge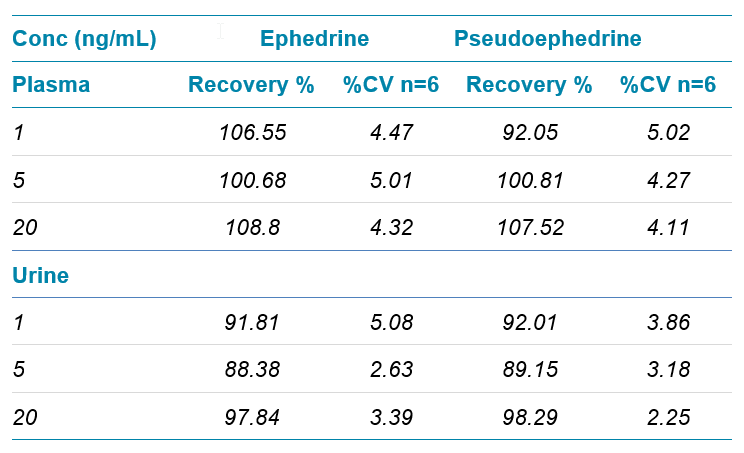 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge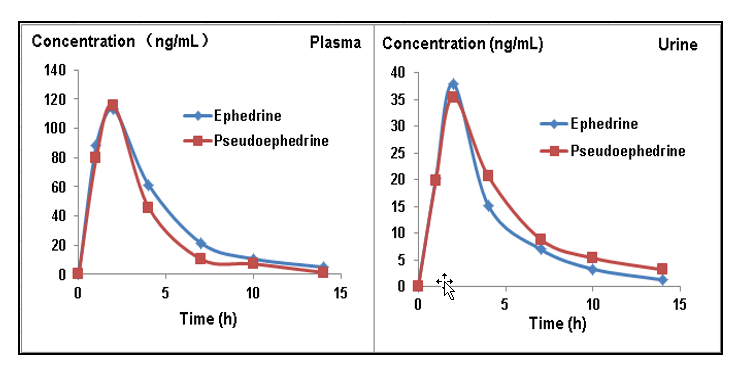 Click to enlarge
Click to enlarge