Click on an episode below to hear first-hand how industry experts have rewritten the rules to overcome significant biopharma analytical challenges.
Get ready to rewrite the rules
Plasmids and AAVs—a special relationship
with Ryan Hylands, Senior Technical Specialist, Analytical Development, Pharmaron
In this episode, we explore the relationship between plasmid DNA (pDNA) and viruses and discuss innovative approaches to analyze pDNA quality.
Recently released episodes
Explore the potential of mRNA
with Jérémie Parot, PhD, Research Scientist, SINTEF
In this episode, we explore the difficulties faced when characterizing mRNA and the opportunities that mRNA research brings to medicine.
Handle with care: Ionizable lipids and impurities
with Adam Crowe, PhD, Sr. Manager, Analytical Development, Precision NanoSystems ULC (part of Cytiva)
In this episode, we take a deep dive into the importance of ionizable lipids in lipid nanoparticles (LNPs) and why new analytical workflows are needed for this expanding field.
Beyond mRNA: Why size of IVT RNA matters
with Rebecca Young, PhD, Sr. Scientist I, RNA Sciences, Aldevron
In this episode, we explore self-amplifying RNA (saRNA), its benefits and how R&D and process development teams utilize this new in vitro transcribed RNA.
Answering the question - what's under that peak?
with Kristen Nields, PhD, Senior Scientist, Janssen
In this episode, we explore the challenges in charge variant analysis and how to address the bottlenecks with new workflow approaches.
Dealing with surprises
with Tyler Heibeck, PhD, Principal Scientist, Sutro Biopharma
In this episode, we talk about the surprises and challenges encountered in drug development and how new analytical approaches may help find the answer.
Finding the spark through collaboration
with Mike McKinnon, Senior Scientist II, Novartis
In this episode, we discuss the challenges of new modalities and how new analytical methods can address the evolving challenges in biotherapeutic characterization.
Change your mindset to move medicine forward
with Anita Liu, Associate Principal Scientist, Merck
In this episode, we take a look at the benefits of intact protein analysis and how to address challenges with new drug modalities.
The team viral vectors
with Jill Bradley-Graham, PhD, Senior Scientist, Gene and Cell Therapy BioAnalytics, Sanofi
In this episode, we discuss why viral vector characterization is particularly challenging and how collaboration is key for newly emerging fields.
The “personality” of oligonucleotides
with Shane Karnik, Senior Laboratory Director, Aliri Bioanalysis
In this episode, we discuss what bioanalysis is and how bioanalysis of oligonucleotides differs from that of other modalities.
Solving the HCP puzzle with mass spectrometry
with Thomas Kofoed, PhD, Co-founder and CEO, Alphalyse
In this episode, we chat about host cell proteins (HCPs), the importance of their analysis in cell and gene therapy products and the challenges of this field.
On or off target? Looking beyond the expected
with Hugo Gagnon, PhD, Chief Scientific Officer, Allumiqs
In this episode, we take a deep dive into what gene editing is and uncover why establishing analytics for gene editing is such a complex task.
Coming soon
Related biopharma applications
Reclaim time for innovation by identifying and advancing drug candidates more confidently with analytical strategies that support your team across modalities, from discovery to commercialization.
Featured products
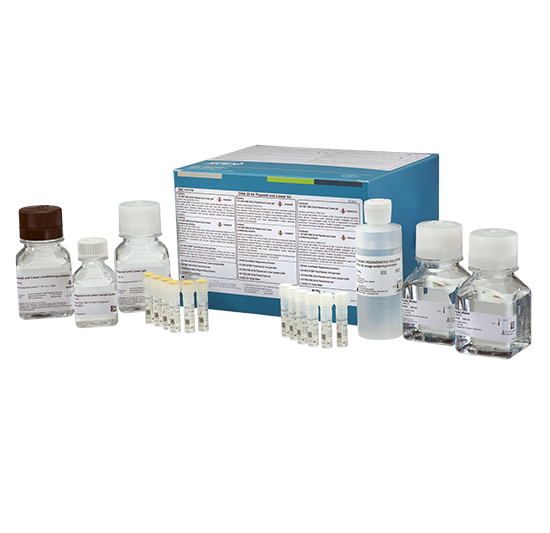
Discover how to automate pDNA purity from 2 to 19 kilobases (kb) and size estimation of linear double-stranded DNA (dsDNA) from 0.1 to 20 kb using the DNA 20 kb Plasmid and Linear kit.
Purpose-built for the biopharmaceutical scientist for efficiency and quality, enabling multiple samples to be run in parallel.
Characterize therapeutic molecules with confidence using this kit-based system.